Definition
Cast iron is a mixture of 2.14% carbon with iron, obtained by thermal heating in blast furnaces to 1200 degrees Celsius. With the help of the sixth element of the periodic table, iron in the form of an alloy acquires increased hardness, losing ductility and malleability, making the material brittle.
In addition to carbon, to obtain special parameters, elements such as Si, Mg, P, S are added to the metal matrix. Alloying agents are also widely used - Cr, V, Ni, Al.
Reduction and carburization of iron in a furnace
All blast furnaces operate on the counterflow principle. At the same time, the following chemical processes alternately occur in them:
- Iron recovery. This process occurs sequentially and looks like this: Fe2O3 - Fe3O4 - FeO - Fe. The reducing agent in this case is carbon monoxide (CO), formed by the interaction of CO2 with hot coke, as well as solid carbon of the latter.
- Carbonization of iron. The reaction in this case looks like this: 3Fe + 2CO = Fe3C + CO2 + Q. Fe3C carbide easily mixes with solid iron, resulting in the formation of an alloy of the latter with carbon. Flowing down, it washes the pieces of coke and carbonizes even more. In addition, substances such as manganese, sulfur, silicon, etc. dissolve in it.
Thus, it becomes clear that blast furnace metal is an alloy of iron with what substance. Cast iron can be obtained simply by carburizing the charge melt.
Story
The technology for making cast iron came to us from China, where cast iron money circulated back in the 10th century AD. The descendants of the Mongols already prepared boilers from this alloy in the 13th century. On the battlefields of the Hundred Years' War, artillery pieces and ammunition cast from this solid solution were used for the first time. In Russia, its widespread use in the manufacture of weapons was established in the 16th century after the appearance of the blast furnace. In this regard, the Ural Iron Foundry was built in 1701, which became the beginning of a folk craft called “Kasli casting”.
Since the 18th century, Great Britain has been the world's leading iron producer. Thanks to Wilkinson's new technology, by the mid-19th century this country produced half the world's total.
Manufacturing technology did not stand still, which allowed the United States to take the lead at the end of the 19th century.
At that time, rails, water and sewer pipes, fireplaces, and such complex engineering structures as bridges began to be made from this alloy.
Ores for smelting
Sand concrete for floor screed: what is it, composition of mixtures
There is quite a lot of iron in the earth's crust, but it is not found in its pure form; it is always mined with rocks in the form of various compounds. Iron ore can be called only those rocks from which it is economically profitable to extract iron by smelting in a furnace. In nature, there are rich and poor iron ores. Speaking from the point of view of the metallurgical industry, the ore contains a number of useful additives that are necessary when producing cast iron - chromium, nickel, manganese and others. There are also harmful inclusions: sulfur, phosphorus, copper, etc. In addition, iron ore can be divided into several groups depending on the mineral:
- red iron ore – 70% iron, 30% oxygen;
- magnetic iron ore – 72.4% iron, 27.6% oxygen;
- brown iron ore – up to 60% iron;
- spar iron ore – up to 48.3% iron.
It would be logical to conclude that blast furnace production of pig iron should involve the use of ore from the second group. But the first one is the most common, which is why it is used more often.
Iron production process
The production of cast iron is carried out in blast furnaces. This process is quite energy-intensive and costly production. 4 main groups of ores are used as raw materials:
- Hematite iron ore, consisting of iron anhydrite oxide, holds 70% (Fe) and 30% (O);
- Magnetite iron ore contains 72.4% (Fe), and 27.6% (O);
- Brown iron ore contains 59.8% elemental iron;
- Siderite iron ore, contains 48.3% (Fe).
The technological process takes place in several stages
First, during the preparation process, iron ore containing iron oxides (FeO and Fe2O3) of at least 40% of the total mass is crushed. Then, by crushing, screening, averaging, washing, enrichment and roasting, they get rid of non-metallic impurities - S, P, As, and increase the mass fraction of the base metal in the ore.
At the end of the preparatory stage, all components are loaded into the oven.
A blast furnace is a continuously operating metallurgical equipment in the form of a shaft, weighing 30 thousand tons. The blast furnace consists of 5 elements: the upper part in the shape of a cylinder - the furnace, the wide conical part - the shaft, the wide part - the steam chamber, the narrowed part - the shoulders and the lower part - the hearth. All components are loaded from above through the furnace, and the finished product and slag come out separately from the bottom of the furnace.
Simultaneously with the ore, coking coals are placed into the blast furnace to act as fuel. During the thermal decomposition of coals, carbon compounds are formed that act as a reducing agent. Fluxes are added to speed up the process of releasing metal from the ore. Typically these are rocks containing oxides of calcium and magnesium.
After the loading stage is completed, the smelting process begins, where the loaded components are converted into an alloy, slag and gas. The physicochemical reactions occurring in this case can be characterized as reduction-oxidation, since the reduction of iron oxides and the oxidation of the reducing agent occurs.
Processes occurring in the furnace
The processes occurring in a blast furnace can be described by the following chemical equations:
When coke is heated, elemental carbon is released, which forms carbon dioxide with oxygen.
C + O2 = CO2 + energy release
CO2, when heated, is further oxidized to carbon monoxide, and reduces elemental iron from its oxides in the ore.
CO2 + C = 2CO
Fe2O3 + 3 CO = 2Fe + 3 CO2
After the reduction reaction, the metal is saturated with carbon, and when it reaches 1150-1200°C, it flows in the form of a metal compound into the furnace. From the remains of empty ore and fluxes, waste is formed - slag, which is continuously removed.
Receiving technology
Cast iron cast in the form of pigs
The source of raw materials for metallurgists is iron ores (rocks with a predominance of iron in the composition).
The ore is sent to processing plants, where part of the “empty” material is removed from the raw material.
The resulting material is transported to the metallurgical plant.
Here they are loaded into blast furnaces:
- Fuel is added - coke (a product of processing coal), limestone, briquetted coal dust.
- Melts at high temperatures.
- During the reduction process, iron with carbon embedded in its structure is obtained from oxides.
As a result of smelting, cast iron and slag are formed (a mixture of fuel ash, unused fluxes, and other products).
Ligatures are added if necessary. They determine the physical and chemical properties of the material.
Production is simple, but environmentally dirty.
Cast iron parameters
Density - 7.2 g/cm3. The melting point is 1200 °C. The fragility and low ductility of the alloy is due to the following factors:
- Increased bond length between Fe atoms due to increased carbon content;
- Incomplete introduction of carbon atoms into the structure of the iron matrix due to the low melting point compared to steel.
It is for these reasons that this solid metal solution has found wide application in the production of parts with high strength. However, it is not suitable for products subject to loads that change rapidly over time.
Structure and properties of cast iron
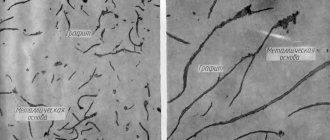
The microstructure of cast iron consists of a metal base (matrix) and graphite inclusions. The properties of cast iron are determined by the properties of the metal base and the nature of graphite inclusions.
Cast irons contain the following structural elements:
- graphite (G);
- perlite (P);
- ferrite (F);
- ledeburite (L);
- phosphide eutectic.
According to the microstructure they are distinguished:
- white cast iron I (C + G);
- gray pearlitic cast iron II (P + G);
- gray ferritic cast iron III (F + G);
- semicircular cast iron II a (P + C + G);
- high-strength cast iron IV (P + nodular graphite).
The formation of the microstructure of cast iron depends on its chemical composition and the cooling rate (thickness) of the casting. The structure of the metal base determines the hardness of cast iron.
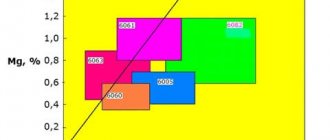
Carbon in cast iron can be present in the form of a chemical compound - cementite Fe 3 C, graphite, or a mixture of both. Compared to the metal base, graphite has low strength. The places where it occurs can be considered as a violation of the continuity of the metal. Cast iron is, as it were, permeated with graphite inclusions, weakening its metal base. As graphite inclusions become rounded (due to the modification of cast iron with SiCa, FeSi, Al, Mg additives), their negative role as cuts in the metal base decreases, and the mechanical properties of cast iron increase.
For example, gray cast iron (a plate form of graphite) has low mechanical properties because the plates containing graphite inclusions act as stress concentrates in the casting. However, gray cast iron has a number of advantages: it has high fluidity and low casting shrinkage; graphite inclusions make the chips brittle, which makes cutting cast iron easier; due to the lubricating effect of graphite, cast iron has good anti-friction properties; dampens vibrations and resonant vibrations well. Critical parts are made from high-strength cast iron (nodular graphite): gears, crankshafts.
Silicon promotes graphitization of cast iron. By changing its composition and the cooling rate of the casting, it is possible to obtain cast iron of various structures.
Manganese prevents graphitization and neutralizes the harmful effects of sulfur, forming refractory MnS compounds with it.
Phosphorus does not have a significant effect on the graphitization process. With an increased phosphorus content, solid inclusions of phosphide eutectic are formed in the structure of cast iron, which increases its casting properties.
Sulfur is a harmful impurity. This causes a deterioration in the casting properties of cast iron, increased shrinkage, increased susceptibility to cracking and a decrease in the red brittleness temperature of cast iron.
Classification of cast irons
There are several types of classification of cast iron.
- Based on elemental carbon content they are divided into:
- hypoeutectic (2.14-4.3%);
- eutectic (4.3%);
- hypereutectic (4.3-6.67%).
- By type of carbon and fracture color:
- White (C > 3%, in carbide form). Its use is limited to the production of products that are not subject to heavy loads due to its significant fragility. But when adding alloying additives containing chromium, nickel, vanadium, aluminum, its performance parameters increase;
- Gray (C -2.5%, in the form of perlite) has good wear resistance and reduces friction. Used in the manufacture of industrial equipment parts subject to cyclic loads. When adding special additives containing Mo, Ni, Cr, B, Cb, Sb, resistance improves when used in aggressive environments;
- Half (C – 3.5-4.2%, in the form of graphite and carbide and the presence of trace amounts of cementite and ledeburite). This type has found its application in the production of products subject to constant friction.
- According to physical parameters, according to GOST 1412-54 and 1215-59, cast iron grades are distinguished:
- Malleable (CC), is its white variety after special firing. At the same time, the proportion of carbon is at the level of 3.5%, and it is presented in the form of Fe2O3 or granular pearlite, with graphite inclusions. Mg, Te, B are usually added as additives to increase friction resistance. It should be noted that this brand is never forged, in the literal sense of the word;
- High-strength (HF), is formed by interspersing spherical carbon inclusions into a metal lattice and introducing magnesium, calcium, selenium, and yttrium into the composition. Characterized by improved mechanical, thermally conductive plastic parameters.
- By specific properties:
- Wear-resistant;
- Antifriction;
- Corrosion resistant;
- Heat resistant;
- Non-magnetic.
- According to the Brinell hardness scale:
- Soft (HB less than 149);
- Moderate hardness (HB 149-197);
- Improved hardness (HB 197-269);
- Hard (HB more than 269).
- According to the value of temporary tensile strength:
- Ordinary strength (less than 20 kgf/mm2);
- Improved strength (20-38 kgf/mm2);
- Maximum strength (more than 38 kgf/mm2).
- According to magnetic characteristics:
- Ferromagnetic - having magnetic properties, due to the high content of ferrite and cementite in the metal matrix;
- Vapor-magnetic – having low magnetic permeability, containing additives of chromium, copper and aluminum.
Marking
According to GOST, all existing brands are designated by 2 letters and 2 numbers, with the numbers reflecting the values of tensile strength (kgf/mm2) and elongation (%). For example, the numbers in the KCh-30-6 brand show a temporary resistance of 30 kgf/mm2 and a relative elongation of 6%.
By introducing special additives into the composition, the composition of the alloy is modified. Then the letter M is added to the brand name.
Wax removal
The question of how to obtain cast iron of good quality also comes down to cleaning it from this undesirable element. Sulfur is the main harmful impurity that significantly worsens the properties of the final smelting product. Its main quantity is contained in coke. Sulfur is removed by increasing the lime (CaO) content in the charge and increasing the temperature in the furnace. The reaction in this case looks like this: FeS + CaO = FeO + CaO + Q. Other methods can be used to reduce the percentage of sulfur content in cast iron. For example, sometimes the already smelted material is processed in a discharge chute or a bowl of soda. In this case, the removal of sulfur occurs as a result of the reaction FeS + NaCO3 = FeO + Na2S + CO2.
Areas of use
The use of different grades of cast iron depends on the metallurgical compound and its performance characteristics.
The white type is used in the production of heating elements and household sanitary ware (baths, sinks), and is also a raw material for the production of malleable varieties of solid solutions.
Gray - is part of various engine components for the engineering industry.
Malleable – in the manufacture of brake pads and parts for industrial grinding equipment. In addition, it has wide application in the textile industry in casting complex shaped spare parts for machinery. KCh is used in the manufacture of kitchen utensils, interior elements, street lamps, and railings for stairs.
The high-strength grade is used in the production of pipes, fittings for water supply, sewerage, and oil production. In addition, sectional radiators are made from it, used in central heating systems of residential buildings and administrative buildings.
Electrical panels and other components of electrical equipment are made from the ferromagnetic type, while its non-magnetic type, on the contrary, is used as an electrical insulating material.
Pig iron is used in huge quantities as a raw material in steel foundries.
Advantages and disadvantages
At the household level, the main advantages of cast iron alloys are: non-toxicity, biocompatibility, hygiene, and heat resistance. Thanks to this, cast iron and other cookware are not destroyed by acid-base compounds (for example, when cooking borscht), are easy to clean, and remain warm for a long time.
For industrialists, other advantages come to the fore:
- A simple, economical way to obtain.
- Durability, preservation of consumer characteristics of products for decades.
- Possibility of producing a wide range.
Plus affordable prices for all products - from ingots to frying pans or a decorative bench.
Cast-iron pan
Disadvantages of the material:
- Fragility.
- Difficulty in welding.
- Vulnerability to corrosion.
- Heaviness of products.
Often, special conditions are required for transportation, assembly, and maintenance of products.
When welding, for example, the parts are heated in advance, the material and mode are selected. That is, they use gas installations, coated or carbon electrodes, and powdered wire.
Interesting Facts
According to Professor Marienbach, cast iron got its name from the Chinese word “zhugun”, which means “foundry worker”.
Cast iron cookware has long been used around the world and is very convenient for preparing various types of food.
An integral attribute of Russian folk tales is the oven, in which, in a cast iron pot - a vessel of a certain shape and cast from a given alloy, the heroes cooked the main dish - jacket potatoes.
The best pancakes are made in a cast iron skillet.
Before the advent of electric irons, housewives used heavy cast iron irons with perfectly smooth soles, heating them until red hot over a fire source.
The next stage was coal cast-iron irons, whose design resembled small stoves. To heat them, birch charcoal was placed inside. This iron even had a pipe to obtain the necessary traction.
The sewer manholes for inspection wells known to us from childhood were round in shape, cast from cast iron and were first made one hundred and fifty years ago.
Pig iron production worldwide in 2015 amounted to more than 898 million tons, which is 3% less than in 2008.
Other indicators
As for the division of cast iron by strength, the following classification is used:
- Normal strength: σв up to 20 kg/mm2.
- Increased strength: σв = 20 - 38 kg/mm2.
- High strength: σв = 40 kg/mm2 and higher.
Based on ductility, cast irons are divided into:
- Non-plastic - relative elongation less than 1%.
- Low plasticity – from 1% to 5%.
- Plastic - from 5% to 10%.
- Increased ductility – more than 10%.
In conclusion, I would also like to be sure to note that even the shape and nature of the pouring has a fairly significant influence on the properties of any cast iron.