06/29/2020 Author: VT-METALL
Issues discussed in the material:
- Brief characteristics of aluminum and its alloys
- Classification of aluminum alloys
- About the marking of aluminum alloys
- 4 ways to process aluminum alloys
- Main groups of aluminum alloys and their properties
- Aluminum alloys with other elements
- Areas of application of aluminum alloys
Aluminum alloys are used much more often than the same metal in its pure form. And this is not surprising: they have much greater strength, as well as resistance to corrosion and high temperatures.
Combinations with various substances endow certain alloys with specific characteristics. Depending on the requirements for the final product, one or more alloying elements are added to aluminum. And to avoid confusion, the resulting alloy is marked in a certain way. That is, the customer can only choose the most suitable metal for his needs.
Brief characteristics of aluminum and its alloys
Aluminum was first obtained by chemists from Denmark (Oersted) and Germany (Wöhler) in 1825 and 1827, respectively. It became possible to produce metal on an industrial scale in 1886 thanks to the developments of the American Charles Hall and the Frenchman Paul Héroult. The cost of aluminum until the end of the 19th century was only slightly inferior to gold.
At the beginning of the last century, aluminum was used only in its pure form. In 1906, the German scientist Wilm thermally strengthened the metal by adding copper (4%), magnesium (0.5%), and manganese (0.5%). This is how the first alloy appeared - duralumin. Aluminum alloys, which, in addition to high strength, have low density, are widely used in industry at present.
The specific strength of aluminum joints (the ratio of tensile strength to density) is significantly higher than that of steels. Due to this, aluminum compounds are widely used in rocket and aircraft construction.
The metal and its alloys are characterized by high manufacturability and ease of deformation, which makes it easy to create parts of complex configurations. The advantages of the material also include corrosion resistance and good electrical conductivity (this characteristic is higher only in silver, copper and gold). The use of aluminum alloys in electronics and electrical engineering is due to the ease of rolling them into foil.
We recommend articles on metalworking
- Steel grades: classification and interpretation
- Aluminum grades and areas of their application
- Defects in metal products: causes and search methods
Due to the low melting point when processing the material, significant energy costs are not required; accordingly, production and products have low costs.
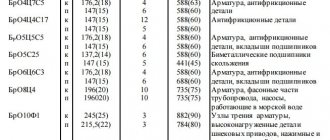
AMg2
Welded and riveted structures, various pipelines, tanks and other medium and lightly loaded products: stained glass windows, room partitions, window and door frames, clock and instrument cases, deck superstructures of ships, wrappers, etc. Rivets are made from wire.
AMgZ
Welded lightly loaded structures, resistant to corrosion: pipelines, tanks, stained-glass windows and partitions, window and door frames, clock and instrument cases, deck superstructures.
AMg5, AMg5 V and AMg6
Welded and riveted structures of medium load, with high corrosion resistance: pipelines, tanks and other similar products.
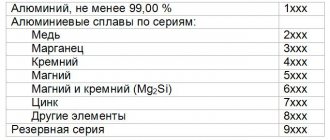
Classification of aluminum alloys
A large number of different characteristics are used to classify aluminum alloys. Depending on the type of auxiliary elements, the following types are distinguished:
- with the addition of additives, which are various materials, for example, magnesium, zinc, chromium, silicon and others.
- with the addition of intermetallic compounds - such compounds contain several metals, for example, copper and magnesium, lithium and magnesium.
Aluminum alloys may contain many elements that give the material certain performance characteristics.
According to the metalworking method, the following types of aluminum compounds are distinguished:
- Wrought aluminum alloys are hard compounds that, due to their high ductility, can be processed by pressing or forging. The performance characteristics of the material are improved by additional processing.
- Foundry - entering production in a liquid state, they are processed after they harden. Case parts of various configurations are made from cast aluminum alloys.
A separate group is represented by technical aluminum, containing less than 1% of foreign impurities. This composition leads to the formation of an oxide film on the surface of the metal, protecting it from the negative effects of the environment. At the same time, the strength characteristics of technical aluminum are quite low.
Depending on the strength of the connection there are:
- super-strong (from 480 MPa);
- medium-strength (from 300–480 MPa);
- low-strength (up to 300 MPa);
A separate group is represented by duralumins, which have special performance properties.
Duralumins
Duralumin belongs to the group of hardenable alloys. It is based on aluminum and copper, as well as additives in the form of iron and silicon. The percentage of the main alloying element ranges from two to seven percent. Moreover, half a percent of copper can be dissolved in aluminum at ambient temperature, and 5.7% at eutectic temperature (548°C).
Heat treatment of duralumin is carried out in several stages. First of all, it must be heated to a value above the limiting solubility line (usually this temperature is about 500°C). This will allow you to achieve the structure of a homogeneous solution of copper in aluminum. The resulting state of the alloy is fixed by instantly cooling it in water at room temperature. This process is called hardening. As a result, a supersaturated solution is formed, characterized by high values of softness and plasticity.
A feature of hardened duralumin is its unstable structure, in which certain transformations occur even under room conditions. Such changes lead to the grouping of excess copper atoms in solution. Moreover, the sequence of arrangement of these atoms is very similar to the order of arrangement of crystals in the CuAl compound. In the crystal lattice of a solid solution, atoms are arranged unevenly, so distortions are formed in it, which contribute to an increase in hardness, improvement in strength properties and deterioration in ductility. At the same time, there is no talk yet about the formation of a chemical compound, as well as about separation from the solid solution. All changes that a hardened alloy undergoes under environmental conditions are called natural aging.
This process is most active during the first hours, and its completion occurs after six days, although in some cases four are enough. When the alloy temperature increases to 150°C, artificial aging occurs. In this case, the alloying time is reduced, but the hardening is not as effective as in the case of natural aging. There is an explanation for this: at elevated temperatures, the diffusion process is faster and easier. This completes the formation phase of the CuAl compound, which is accompanied by its separation from the solid solution. The strengthening effect is not as significant as in the case of distortion of the structure of the hard alloy by natural aging.
If we compare the results of duralumin aging carried out under different conditions, it becomes extremely clear that the strength characteristics of the metal can be increased most effectively with natural aging for four to six days.
Marking of aluminum alloys
When determining the grade of aluminum alloys, you may encounter certain difficulties. Marking is carried out in such a way that no questions arise when clarifying the connection. The compositions have a specific alphanumeric designation.
Features of the marking are as follows:
- at the beginning there are one or more letters indicating the composition of the compound;
- markings include a digital serial number;
- The marking may also end with a letter indicating the characteristics of the material processing (for example, thermal).
Let's get acquainted with the marking rules using the example of the D17P alloy. The first letter D indicates the composition of the alloy - duralumin. All duralumin contains certain chemical elements that differ in quantitative content. Serial number 17 indicates a specific material with certain properties. The letter P at the end of the marking is used to indicate the method of processing a semi-hardened joint obtained under pressure without preheating the metal; accordingly, the strength characteristics will be half of the maximum possible.
VT-metall offers services:
Marking of aluminum alloys is carried out in accordance with GOST 4784-97, which defines the basic requirements for the designation of connections.
Main groups of aluminum alloys and their properties
To work with aluminum and its compounds, it is necessary to become familiar with the properties of the metal, since they significantly affect the scope of application of the parts and the characteristics of the material. Earlier we talked about the classification of aluminum alloys.
Next, we will talk about the most common types of metal and their properties.
- Alloys with aluminum, copper and silicon.
The compound is also known as alcusine. Alloys containing copper and silicon are used to make parts of industrial equipment. Excellent technical properties allow them to be used under constant load conditions.
- Aluminum-copper alloys.
The technical characteristics of compositions containing copper are comparable to low-carbon steels. The main disadvantage is poor corrosion resistance. The parts are coated with a protective compound that protects them from the negative effects of the environment. To improve the qualities of the material, alloying components (manganese, iron, magnesium and silicon) are used.
- Aluminum-silicon alloys.
These compounds are called silumin and are used for the production of decorative elements. To improve the characteristics of aluminum alloys, sodium and lithium are used.
- Aluminum-magnesium alloys.
The presence of magnesium in the composition increases the strength characteristics of the material and also facilitates the welding process. The magnesium content should not exceed 6%. A higher percentage will reduce the anti-corrosion properties of the joint. To increase strength without reducing corrosion resistance, manganese, vanadium, chromium or silicon are added to the compositions. Each additional percentage of magnesium improves strength by 30 MPa.
- Aluminum-manganese alloys.
To increase corrosion resistance, manganese is added to the compound. Thanks to it, the strength and weldability of the material are increased. In addition to manganese, iron and silicon are added to the composition.
- Alloys with aluminum, zinc and magnesium.
Aluminum alloys with magnesium and zinc are distinguished by their high strength characteristics and ease of processing. To improve the properties of the material, it is subjected to heat treatment. The disadvantage of such compounds is their low corrosion resistance. To correct this disadvantage, an alloying component is used - copper.
- Avial.
These alloys, in addition to aluminum, contain magnesium and silicon. The connections are characterized by high ductility and corrosion resistance.
More magnesium
However, in alloys with high magnesium content there is a tendency for the intermetallic phase Mg5Al8 to form at grain boundaries and in regions of localized deformation within the microstructure. This is because the equilibrium solubility of magnesium in aluminum is only about 2%. The precipitation of excess phase in this case is equivalent to what occurs in age-hardening alloys, but with a negative effect on the properties of the alloy. Particle precipitation occurs slowly at room temperature, but accelerates with increasing temperature or if the alloy is subjected to severe cold plastic deformation. This phenomenon makes the alloy susceptible to certain types of intergranular corrosion, such as stress corrosion, and/or deterioration of mechanical properties during service at elevated temperatures.
Aluminum alloys with other elements
Alloying elements used in the manufacture of aluminum alloys and improving their quality characteristics are also the following.
Beryllium reduces oxidation during heat treatment. The low beryllium content (0.01–0.05%) improves the fluidity of aluminum compounds used in the production of internal combustion engine parts (pistons and cylinder heads).
Bismuth, as well as lead, tin or cadmium, which have a low melting point, when added to alloys, facilitate the process of cutting metal. These components contribute to the formation of soft, fusible phases, which ensure chip brittleness and cutter lubrication.
Compounds with added gallium (0.01–0.1%) are used to produce sacrificial anodes.
A small amount of iron (no more than 0.04%) is added to the material used to make wires, thereby increasing the strength and creep of the material. In addition, iron reduces the adhesion of the composition to the walls of molds when casting into a chill mold.
Magnesium in aluminum
The introduction of magnesium into aluminum in an amount of up to 6% as the main alloying element results in solid solution strengthening of the alloy and high efficiency of strain hardening. This provides the 5xxx series alloys with fairly high strength properties - higher than those of the 3xxx series alloys - while maintaining good formability.
With a certain susceptibility to intergranular corrosion (with a magnesium content of more than 3%), these alloys have good corrosion resistance, especially corrosion resistance in sea water and sea atmosphere, which is significantly higher than that of other alloy series.
Areas of application of aluminum alloys
Aluminum alloys are widely used in many fields. Their performance characteristics make them one of the five most common metal compounds.
First, due to their lightness and strength, they began to be used in the production of airships and aircraft.
Currently, due to the high melting point, aluminum compounds are used in the production of high-speed trains. The surface heats up while moving at high speed, but does not undergo deformation.
The metal and its compounds are widely used in shipbuilding, where they are preferred over steel. Aluminum hulls are not subject to fouling by shells, which negatively affect the streamlining of ships and their speed. Cleaning a steel hull requires significant time and money. Thus, the initial investment in the construction of an aluminum housing is recouped through cheaper operation.
The low cost and low specific weight ensured the demand for the material in the military industry; for example, it is used to produce individual elements of small arms. Rocket fuel is made using aluminum compounds.
High electrical conductivity is due to the use of aluminum alloys for the production of wires and parts of radio receivers. They are suitable for the manufacture of various dimensional electrical conductors (power lines, high-voltage cable sheaths, switchgear busbars), which is caused by their noticeable advantages over other metals. For example, aluminum cable sheaths are characterized by greater strength and lower density than lead ones. Countries with highly developed industries spend about 15% of aluminum to meet electrical needs.
The metal currently continues to be used for the production of tableware. Aluminum forks, spoons, pots and containers for liquids continue to be in demand.
Aluminum has also found application in the food industry as a food additive. The letter E is used to designate the composition of aluminum products. The metal acts as a dye in confectionery products and protects products from mold. Various products are packaged in thin aluminum foil, the thickness of which does not exceed 0.009 mm. And aluminum strip with a thickness of 0.2-0.3 mm is used for the production of cans.
One specific use case for aluminum alloys is nuclear reactors. Most of them use thermal neutrons during operation. Accordingly, the reactor design should consist of metals that weakly absorb such particles. For example, from aluminum, which is also characterized by high corrosion resistance when exposed to hot water, superheated steam, carbon dioxide, which most often act as a heat source in reactors.
Metal connections
Alloys are obtained by artificially adding other metals to aluminum in order to obtain the necessary properties. And today there is an endless number of compositions of such alloys that have the widest application.
- The most famous area of their application is aircraft manufacturing. For the production of aircraft, alloys consisting of aluminum, zinc and magnesium are used, which results in a super-strong and reliable material.
- Alloys of aluminum with iron, titanium, and nickel are also often used.
If you want to make something out of aluminum yourself, the following video will tell you how to melt it at home: