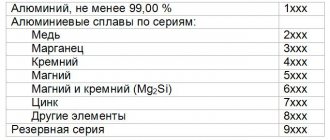
Aluminum, as the lightest and most ductile metal, has a wide range of uses. It is resistant to corrosion, has high electrical conductivity, and can easily withstand sudden temperature fluctuations. Another feature is that upon contact with air, a special film appears on its surface, which protects the metal.
All these, as well as other features, contributed to its active use. So, let's find out in more detail what the uses of aluminum are.
Consumption in industry and life
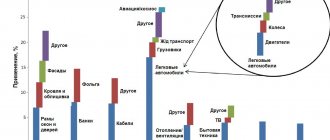
The figure below shows eight industrial and construction sectors in which aluminum is being used particularly strongly. The percentage shares of various industrial sectors in total consumption are presented according to statistics from the International Aluminum Institute for 2007. Since then, I think the picture as a whole has not changed, and these data are quite relevant.
Application of aluminum in finished industrial products [1]
The main industries that actively use aluminum are:
- Construction
- Product packaging
- Electrical industry
- Transport engineering
- Manufacturing of machinery and equipment
- Production of goods for everyday life
- Powder metallurgy
- Deoxidation of steel in ferrous metallurgy
How to use the basic properties of aluminum in construction
Construction is one of the main aluminum consuming industries. 25% of all metal produced is used in it. The modern appearance of megacities would be impossible without the use of aluminum. It makes it possible to create functional and beautiful buildings that strive upward. Skyscrapers of office centers have glass facades mounted on durable, lightweight aluminum frames.
Modern shopping, entertainment and exhibition centers are based on aluminum frames. Structures made from this metal are used for the construction of swimming pools, stadiums and other sports buildings. Aluminum is one of the most popular materials among architects, builders, and metal designers. Why? Let's figure it out.
Aluminum is a strong and lightweight metal that is resistant to corrosion, has a long service life and is completely non-toxic. It can be easily processed, welded, soldered, simply drilled, sawed, tied and connected with screws. This metal can take any shape through extrusion. Aluminum will help realize the most daring plans of the architect. It is used to make structures that cannot be made from other materials: plastic, wood or steel.
Construction
Aluminum windows and facades
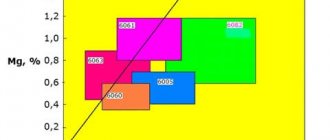
The main aluminum alloys that are used in the construction industry are alloys 6063 and 6060, as well as alloy 6082 (in Europe) and alloy 6061 (in North America). They have fairly high strength (6082 and 6061 - up to 400 MPa) and good corrosion resistance.
Window aluminum profiles with thermal decoupling (alloys 6060/6063)
The most important structural characteristics of aluminum, which determine the use of aluminum as a material for window and door frames:
- strength for rigidity and safety;
- the ability to take complex shapes (provided by extrusion);
- attractive appearance;
- corrosion resistance;
- minimal need for maintenance.
Curtain facade with aluminum frame (alloys 6060/6063)
Post-transom facade
Aluminum roofing and aluminum cladding of buildings
Decorative and protective profiled cladding materials are often made from rolled aluminum sheets. Various types of decorative and protective coatings make them ideal materials for use as roofing materials.
Application for roofing and cladding of buildings provides the following properties of aluminum:
- low weight due to low density;
- resistance to water;
- corrosion resistance;
- decorative look.
Aluminum roofing
Metal connections
Alloys are obtained by artificially adding other metals to aluminum in order to obtain the necessary properties. And today there is an endless number of compositions of such alloys that have the widest application.
- The most famous area of their application is aircraft manufacturing. For the production of aircraft, alloys consisting of aluminum, zinc and magnesium are used, which results in a super-strong and reliable material.
- Alloys of aluminum with iron, titanium, and nickel are also often used.
If you want to make something out of aluminum yourself, the following video will tell you how to melt it at home:
Source
Transport
Aluminum in passenger cars
The average weight of aluminum in passenger cars in Europe in 2006 was about 118 kg and continued to increase. Its share in various components and parts of cars is (in kilograms per car):
- engine cylinder blocks: 40.3
- transmission: 16.3
- Chassis, suspension and handling: 12.5
- wheels: 17.7
- heat exchanger: 12.3
- brakes: 3.7
- body: 6.8
- heat shields: 1.4
- bumpers: 2.8
- other components: 3.9.
Aluminum car engine block
Aluminum car wheel rim
The use of aluminum for the manufacture of automotive parts is due to its following properties:
- low density;
- strength;
- rigidity;
- viscosity;
- price;
- corrosion resistance.
Aluminum car frame
Aluminum alloys for trucks
Aluminum alloys for automobile tanks [5]
Production of aluminum automobile tanks [5]
Aluminum alloys for dump truck bodies [5]
Production of aluminum dump truck bodies [5]
Aluminum alloys for car vans [5]
Aluminum alloys for truck chassis [5]
Aluminum in carriage building
Construction of the Intercity Express high-speed train from extruded aluminum profiles - Germany, 1992
Aluminum urban rail car [7]
Aluminum freight car for coal transportation [7]
Aluminum in shipbuilding
Aluminum patrol boat
Cruise ship with aluminum superstructure [5]
Aluminum catamaran yacht [5]
Aluminum alloys for aircraft
The Wright brothers' first airplane in 1903 was primarily made of wood with an aluminum engine.
Among the aluminum alloys used in aircraft construction, high-strength wrought alloys dominate, such as alloy 2024 (containing copper and magnesium) and alloy 7075 (containing magnesium, zinc and some copper). Most aluminum alloys used in aircraft construction are non-weldable and are joined mainly with rivets.
The figures below show the use of 2xxx series alloys for the manufacture of aircraft fuselage and 7xxx series alloys for wings.
(a)
(b)
The use of aluminum alloys in aircraft construction: a – alloys of the 7xxx series for the fuselage and b – alloys of the 2xxx series for the wings [2].
Airbus A380
Basic requirements for aluminum alloys in the aerospace industry:
- low density;
- high strength;
- precision machining;
- corrosion resistance;
- price.
Production of aluminum.
The documented discovery of aluminum occurred in 1825. This metal was first obtained by the Danish physicist Hans Christian Oersted, when he isolated it by the action of potassium amalgam on anhydrous aluminum chloride (obtained by passing chlorine through a hot mixture of aluminum oxide and coal). Having distilled off the mercury, Oersted obtained aluminum, although it was contaminated with impurities. In 1827, the German chemist Friedrich Wöhler obtained aluminum in powder form by reducing hexafluoroaluminate with potassium:
Na3AlF6 + 3K ® Al + 3NaF + 3KF. Later he managed to obtain aluminum in the form of shiny metal balls. In 1854, the French chemist Henri Etienne Saint-Clair Deville developed the first industrial method for producing aluminum - by reducing the melt of tetrachloroaluminate with sodium: NaAlCl4 + 3Na ® Al + 4NaCl. However, aluminum continued to be an extremely rare and expensive metal; it was not much cheaper than gold and 1500 times more expensive than iron (now only three times). A rattle was made from gold, aluminum and precious stones in the 1850s for the son of French Emperor Napoleon III. When a large ingot of aluminum produced by a new method was exhibited at the World Exhibition in Paris in 1855, it was looked upon as if it were a jewel. The upper part (in the form of a pyramid) of the Washington Monument in the US capital was made from precious aluminum. At that time, aluminum was not much cheaper than silver: in the USA, for example, in 1856 it was sold at a price of 12 dollars per pound (454 g), and silver for 15 dollars. In the 1st volume of the famous Brockhaus Encyclopedic Dictionary published in 1890, Efron said that “aluminum is still used primarily for the manufacture of... luxury goods.” By that time, only 2.5 tons of metal were mined annually throughout the world. Only towards the end of the 19th century, when an electrolytic method for producing aluminum was developed, its annual production began to amount to thousands of tons, and in the 20th century. – million tons. This transformed aluminum from a semi-precious metal to a widely available metal.
The modern method of producing aluminum was discovered in 1886 by a young American researcher, Charles Martin Hall. He became interested in chemistry as a child. Having found his father's old chemistry textbook, he began to diligently study it and carry out experiments, once even receiving a scolding from his mother for damaging the dinner tablecloth. And 10 years later he made an outstanding discovery that made him famous throughout the world.
As a student at age 16, Hall heard from his teacher, F. F. Jewett, that if someone could develop a cheap way to produce aluminum, that person would not only do a great service to humanity, but also make a huge fortune. Jewett knew what he was saying: he had previously trained in Germany, worked with Wöhler, and discussed with him the problems of producing aluminum. Jewett also brought a sample of the rare metal with him to America, which he showed to his students. Suddenly Hall declared publicly: “I will get this metal!”
Six years of hard work continued. Hall tried to obtain aluminum using different methods, but without success. Finally, he tried to extract this metal by electrolysis. At that time there were no power plants; current had to be generated using large homemade batteries from coal, zinc, nitric and sulfuric acids. Hall worked in a barn where he set up a small laboratory. He was helped by his sister Julia, who was very interested in her brother’s experiments. She preserved all his letters and work journals, which make it possible to literally trace the history of the discovery day by day. Here is an excerpt from her memoirs:
“Charles was always in a good mood, and even on the worst days he was able to laugh at the fate of unlucky inventors. In times of failure, he found solace at our old piano. In his home laboratory he worked for long hours without a break; and when he could leave the set up for a while, he would rush across our long house to play a little... I knew that, playing with such charm and feeling, he was constantly thinking about his work. And music helped him with this.”
The most difficult thing was to select an electrolyte and protect the aluminum from oxidation. After six months of exhausting labor, several small silver balls finally appeared in the crucible. Hall immediately ran to his former teacher to tell him about his success. “Professor, I got it!” he exclaimed, holding out his hand: in his palm lay a dozen small aluminum balls. This happened on February 23, 1886. And exactly two months later, on April 23 of the same year, the Frenchman Paul Héroux took out a patent for a similar invention, which he made independently and almost simultaneously (two other coincidences are also striking: both Hall and Héroux were born in 1863 and died in 1914).
Now the first balls of aluminum produced by Hall are kept at the American Aluminum Company in Pittsburgh as a national relic, and at his college there is a monument to Hall, cast from aluminum. Jewett subsequently wrote: “My most important discovery was the discovery of man. It was Charles M. Hall who, at the age of 21, discovered a method of reducing aluminum from ore, and thus made aluminum that wonderful metal which is now widely used throughout the world.” Jewett's prophecy came true: Hall received wide recognition and became an honorary member of many scientific societies. But his personal life was unsuccessful: the bride did not want to come to terms with the fact that her fiancé spends all his time in the laboratory, and broke off the engagement. Hall found solace in his native college, where he worked for the rest of his life. As Charles’s brother wrote, “College was his wife, his children, and everything else—his whole life.” Hall bequeathed the majority of his inheritance to the college - $5 million. Hall died of leukemia at the age of 51.
Hall's method made it possible to produce relatively inexpensive aluminum on a large scale using electricity. If from 1855 to 1890 only 200 tons of aluminum were obtained, then over the next decade, using Hall’s method, 28,000 tons of this metal were already obtained worldwide! By 1930, global annual aluminum production reached 300 thousand tons. Now more than 15 million tons of aluminum are produced annually. In special baths at a temperature of 960–970 ° C, a solution of alumina (technical Al2O3) in molten cryolite Na3AlF6, which is partially mined in the form of a mineral, and partially specially synthesized, is subjected to electrolysis. Liquid aluminum accumulates at the bottom of the bath (cathode), oxygen is released at the carbon anodes, which gradually burn. At low voltage (about 4.5 V), electrolysers consume huge currents - up to 250,000 A! One electrolyzer produces about a ton of aluminum per day. Production requires a lot of electricity: it takes 15,000 kilowatt-hours of electricity to produce 1 ton of metal. This amount of electricity is consumed by a large 150-apartment building for a whole month. Aluminum production is environmentally hazardous, since the atmospheric air is polluted with volatile fluorine compounds.
Space technology
The first person to understand the enormous potential of aluminum for space was the great novelist Jules Verne. In his novel “A Trip to the Moon,” he described in detail an aluminum rocket back in 1865.
Aluminum alloys for spacecraft
The body of the first Soviet satellite, which was launched in October 1957, was made of aluminum-magnesium alloy AMg6 with a magnesium content of 6%. Aluminum-magnesium alloys remain the main material for the manufacture of rocket bodies. Duralumin aluminum alloys are also used in the internal compartments of missiles.
The first artificial space object - Soviet Sputnik 1
In the last decades of the 20th century, aluminum-lithium alloys began to be used in spacecraft. The density of lithium is only 0.533 g/cm3 - it is lighter than water. Additions of lithium to aluminum in amounts up to 2.5% reduce the density of the aluminum alloy and also increase its elastic modulus. Thus, alloy 8090 has a density 10% lower and a modulus of elasticity 11% higher than that of alloys 2024 and 2014, popular in aircraft construction. The figure below shows the Curiosity rover wheel made of aluminum alloy 7075.
Curiosity rover wheel made of aluminum alloy 7075-T7351
Aluminum is also used as a binder material in boron-aluminum composites, which are currently also used in space technology.
Boron-aluminum composite (40% boron fibers)
Powdered aluminum - a component of rocket fuel
The high chemical activity of aluminum makes it possible to use it as a component of rocket fuel for solid rocket boosters in the space launch system (SLS) being developed by NASA.
In rocket boosters, aluminum powder and ammonia perchlorate are bonded together using a special binder. This mixture, similar to eraser material, is then placed in a steel casing [3].
When this mixture ignites, the oxygen from the ammonia perchlorate combines with the aluminum to form aluminum oxide, aluminum chloride, water vapor and nitrogen gas, and release enormous amounts of energy.
Aluminum is a component of solid fuel for NASA rocket boosters [3]
How to use the basic properties of aluminum
Aluminum in its pure form has weak mechanical properties. That is why its alloys are most often used.
There are quite a lot of such alloys, here are the main ones:
- aluminum with manganese;
- duralumin;
- aluminum with magnesium;
- aluminum with copper;
- avial;
- silumins.
These alloys are based on aluminum; they differ only in additives. The latter make the material durable, easy to process, and more resistant to wear and corrosion.
There are several main applications for aluminum (pure or alloyed). Made from metal:
- foil and wire for household use;
- dishes;
- sea and river vessels;
- aircraft;
- reactors;
- spacecraft;
- architectural and construction elements and structures.
Aluminum is one of the most important metals along with iron and its alloys. These two elements of the periodic table are most widely used by humans in their activities.
Product packaging
Rolled aluminum – strips and foil – are used in the packaging of bulk and liquid products. Aluminum packaging accompanies us everywhere in our lives - for example, aluminum cans and bottles, foil in food and medicine packaging. Aluminum has low density, food and beverage compatibility, and an attractive appearance. This makes it an ideal material for various types of packaging: hard (cans) and soft (foil).
Aluminum cans for food packaging [6]
Aluminum cans
Aluminum is used to make 75% of beverage cans and 15% of aerosol containers. Aluminum cans provide a significant reduction in packaging weight compared to similar steel cans.
The can body is made of 3000 series alloy (aluminum-manganese alloys), which, after deep upsetting, is rolled out to a wall thickness of 0.27 mm.
The lid of the jar makes up 25% of its weight. It is made from a more durable aluminum-magnesium alloy. The “opener” lever built into the can, which is attached to the can with an integral rivet, consists of a different aluminum-magnesium alloy. This rivet is rolled from the body of the cap during its manufacture.
Aluminum can for packaging beer and soft drinks
Requirements for aluminum alloys for the packaging industry:
- low density;
- strength;
- good formability;
- compatibility with food and drinks;
- decorativeness (the ability to apply drawings and inscriptions);
- price.
Packaging foil
Aluminum foil is usually made from 1000 series grades of commercial aluminum. The properties of aluminum that make it suitable for use as a foil material are as follows:
- strength and impermeability to liquids and gases with small thickness;
- low density;
- thermal conductivity;
- heat resistance;
- resistance to penetration of gases and liquids;
- compatibility with food and drinks;
- aesthetic and decorative potential.
Aluminum Packaging Foil
Basic physical properties of aluminum
The main characteristics of aluminum are high electrical and thermal conductivity, ductility, resistance to cold and corrosion. It can be processed by rolling, forging, stamping, drawing. Aluminum is highly weldable.
Impurities present in the metal in varying quantities significantly worsen the mechanical, technological and physicochemical properties of pure aluminum. The main ones are titanium, silicon, iron, copper and zinc.
According to the degree of purification, aluminum is divided into technical metal and high purity. In practice, the differences between these types lie in their resistance to corrosion in different environments. The cost directly depends on the purity of the aluminum. Technical metal is suitable for the production of rolled products, various alloys, cable and wire products. Pure is used for special purposes.
Aluminum has high electrical conductivity, second only to gold, silver, and copper. However, the combination of this indicator with low density allows it to be used in the production of cable and wire products on a par with copper. The electrical conductivity of a metal can increase during prolonged annealing or deteriorate during cold-working.
By increasing the purity of aluminum, manufacturers increase its thermal conductivity. Impurities of copper, manganese and magnesium can reduce this property. Only copper and silver have higher thermal conductivity. It is thanks to this property that this metal is used for the production of cooling radiators and heat exchangers.
The specific heat of aluminum, as well as its melting point, is quite high. These indicators significantly exceed similar values for most metals. As the purity of the metal increases, its ability to reflect light rays from the surface also increases. Aluminum is highly polishable and anodizes well.
The metal is close in properties to oxygen; its surface in air is quickly covered with a thin and durable film of aluminum oxide. Possessing anti-corrosion properties, it protects the metal from rust formation and prevents further oxidation. Aluminum does not interact with nitric acid (concentrated and diluted) and organic acids, it is resistant to fresh and salt water.
These features of aluminum make it resistant to corrosion, which is what people use. That is why it is especially widely used in construction. Interest in it is also increasing because of its lightness combined with strength and softness. Not every substance has such characteristics.
In addition to the above, aluminum has several more interesting physical properties:
- Malleability and ductility - aluminum has become a material for the manufacture of strong and lightweight thin foil, as well as wire.
- Melting occurs at a temperature of +660 °C.
- Boiling point +2 450 °C.
- Density – 2.7 g/cm³.
- The presence of a volumetric face-centered metal crystal lattice.
- Connection type: metal.
Wires and cables
The high electrical conductivity of the 1000 series aluminum grades, as well as the 8000 series aluminum alloys, makes them very suitable for the manufacture of electrical conductors. Aluminum conductors are used in the following cases:
- electrical distribution substations;
- power systems of high-rise buildings;
- high-voltage power lines;
- most underground power lines;
- power cables for industrial applications.
Most aluminum in the electrical industry is used in the form of cables (8 out of 13%). However, it is also used in the form of electrical busbars for equipment with high current strength, as well as for powering large buildings. In addition, cables for industrial, commercial and residential buildings may contain many insulated conductors, which are placed in a common protective aluminum sleeve.
Requirements for aluminum used for electrical applications:
- reasonable cost;
- sufficiently high electrical conductivity;
- corrosion resistance;
- strength.
How was aluminum discovered and what are its main properties?
Aluminum is a paramagnetic metal, quite light, with a silvery color. It lends itself well to machining and casting, and is easy to mold. In the earth's crust, this element is the third most abundant, ahead only of oxygen and silicon. Our subsoil contains as much as 8% of this metal, which is significantly more than gold, the amount of which is no more than five millionths of a percent.
Aluminum is actively used in most areas of production. Its alloys are used for the manufacture of household appliances, transport, mechanical engineering and electrical engineering. Capital construction also cannot do without it.
VT-metall offers services:
It is extremely common in the earth's crust, being the first of the metals and the third chemical element (the first place is for oxygen, the second is for silicon). The share of aluminum in our subsoil is 8.8%. The metal is part of a large number of rocks and minerals, the main of which is aluminosilicate.
In the form of compounds, aluminum is found in basalts, feldspars, granites, clay, etc. However, it is mainly obtained from bauxites, which are quite rarely found in the form of deposits. In Russia, such deposits are found only in the Urals and Siberia. On an industrial scale, aluminum can also be mined from nephelines and alunites.
We recommend articles on metalworking
- Steel grades: classification and interpretation
- Aluminum grades and areas of their application
- Defects in metal products: causes and search methods
Animal and plant tissues contain aluminum as a trace element. Some organisms, for example, mollusks and mosses, are its concentrators, accumulating it in their organs.
Humanity has been familiar with an aluminum compound called potassium alum for a long time. It was used in the process of tanning leather, as a means that, when swelling, binds the various components of the mixture. In the second half of the 18th century. scientists discovered aluminum oxide. But the substance was obtained in its pure form much later.
This was first achieved by C.K. Oersted, who isolated aluminum from chloride. While conducting the experiment, he treated potassium salts with amalgam, as a result of which a gray powder emerged, recognized by all as pure aluminum.
Subsequently, by studying the metal, scientists determined its chemical properties, manifested in its high ability to recover and activity. That is why they did not work with aluminum for a long time.
But already in 1854, the French scientist Deville, using electrolysis of the melt, managed to obtain metal in ingots. This method is still used today. Aluminum began to be produced on an industrial scale at the beginning of the 20th century, when enterprises were able to gain access to large amounts of electricity.
Today, aluminum is one of the most used metals in the production of household appliances and construction.
cars and equipment
Heating and ventilation systems
Aluminum alloys of the 3000, 5000 and 6000 series have good thermal conductivity. Combined with high strength, these alloys are a good choice for heating and ventilation applications. These systems include the following components using aluminum alloys:
- compressors;
- condensers/evaporators;
- expansion valves;
- fans;
- pipes.
Properties of aluminum that are important for heating and ventilation systems:
- high thermal conductivity;
- high contact coefficient;
- low density;
- high corrosion resistance.
Basic chemical properties of aluminum
From a chemical point of view, aluminum is an extremely strong reducing agent, capable of being a highly active substance in its pure form. The main condition is to remove the oxide film.
Aluminum is capable of reacting with:
- alkaline compounds;
- acids;
- gray;
- halogens.
Aluminum does not interact with water under normal conditions. Iodine is the only halogen with which the metal reacts without heating. To interact with others, an increase in temperature is required.
Let's look at a few examples showing the chemical properties of this metal. These are equations illustrating the interaction with:
- alkalis: 2Al + 6H2O + 2NaOH = Na[Al(OH)4] + 3H2;
- acids: AL + HCL = AlCL3 + H2;
- gray: 2AL + 3S = AL2S3;
- halogens: AL + Hal = ALHal3.
The main property of aluminum is its ability to restore other substances from their compounds.
The reactions of its interaction with oxides of other metals clearly demonstrate all the reducing properties of the substance. Aluminum perfectly separates metals from various compounds. An example would be: Cr2O3 + AL = AL2O3 + Cr.
The metallurgical industry actively uses this ability of aluminum. The method of obtaining substances, which is based on this reaction, is called aluminothermy. The chemical industry uses aluminum most often to produce other metals.
Consumer goods
Aluminum is used in large quantities in the manufacture of various components, parts and housings of many consumer products that surround our lives - household products, for example, refrigerators, freezers, dishwashers. Refrigerators and freezers contain refrigeration units, which, as mentioned above, also contain significant amounts of aluminum. Important properties of aluminum for consumer products are:
- aesthetic properties;
- corrosion resistance;
- strength;
- high thermal conductivity (for refrigeration units).
Steel deoxidation
To remove oxygen from molten steel, so-called deoxidizers are added to the melt. Aluminum, as well as manganese and ferrosilicon, are often used as deoxidizing agents.
Typically, aluminum is added to molten steel in the form of 10 kg bars. To deoxidize one ton of steel, about one kilogram of aluminum is required. Aluminum grades for deoxidation are established by GOST
Properties of aluminum that determine its use in the metallurgical industry as a steel deoxidizer:
- reacts actively with oxygen in molten steel;
- price;
- influence on steel metallurgy.
Source
Medicine
Equipment and tools
Anodized aluminum is widely used for products and parts in medical and dental equipment, including:
- Interior decoration of hospital wards and medical offices
- Instruments that can withstand regular autoclave sterilization
- Hospital beds, stretchers, strollers and other means for moving patients
- Medical Oxygen Equipment
- Dental equipment and instruments
- X-ray machines
- Dialysis equipment.
Medicine packaging
Aluminum foil is an unrivaled barrier that reliably protects medications from microorganisms, sunlight, oxygen and other gases. Therefore, this foil is the main material for protective packaging of medicines and pharmaceutical materials.
Medicinal tablets in aluminum packaging
The effect of aluminum on the human body
Aluminum in medicine
Despite the fact that aluminum in large quantities is harmful to human health, it is widely used in the treatment of a number of diseases.
Aluminum-based preparations are made that have enveloping, analgesic, adsorbent and antacid effects. The antacid properties of aluminum are used to reduce the acidity of gastric juice, since it very actively binds to hydrochloric acid. The indication in this case may be, for example, gastritis with high acidity (hyperacid gastritis). Aluminum preparations find both internal and external use.
The role of aluminum in the human body.
Aluminum plays a very important role - it takes part in the process of regeneration (restoration) of epithelial and connective tissues, maintaining bone strength, and in the formation of peptides and phosphate complexes. Aluminum affects the function of the parathyroid glands and has both an activating and an inhibitory effect on digestive enzymes. A person consumes aluminum with such products as: oat flakes, rye grains, wheat grains, flour, peas,
rice cereal, potatoes, kiwi, cabbage, carrots, apples.
Lack of aluminum in the human body
Microelement deficiency in the body is such a rare phenomenon that the likelihood of its development is reduced to zero.
Every year the amount of aluminum in the human diet is rapidly increasing.
The compound comes in food, water, food additives (sulfates), medications, and sometimes in the air. In medical practice throughout history, several isolated cases of deficiency of the substance in the human body have been recorded. Thus, the pressing problem of the 21st century is the oversaturation of the daily menu with an element rather than the development of its insufficiency.
Despite this, let’s look at the consequences of aluminum deficiency in the body.
- General weakness, loss of strength in the limbs.
- Slowing growth and development of children and adolescents.
- Impaired coordination of movements.
- Destruction of cells, tissues and loss of their functionality.
These deviations occur if a person regularly does not receive the daily requirement of aluminum (30-50 micrograms). The poorer the diet and the lower the intake of the compound, the more intense the symptoms and consequences of deficiency.
Excess aluminum is hazardous to health
Excess microelement is toxic.
An increased aluminum content is dangerous for human health, since immunity is reduced, and sometimes irreversible changes occur in the body, which sharply reduce life expectancy.
Characteristic signs of an excess of microelement: decreased hemoglobin, decreased number of red blood cells in the blood, cough, loss of appetite, constipation, nervousness, mental disorders, speech disorders, spatial orientation, clouding of mind. memory loss, convulsions.
Remember, aluminum belongs to the category of immunotoxic microelements, so to maintain health you need to monitor the amount of the compound entering the body daily.
If aluminum is considered an immunotoxic element for the human body, we decided to find out how aluminum enters the human body.
Practical part: iron
Experiment “Study of iron content in apples.”
First I conduct an experiment on a red apple. I cut a red apple in half and examined the cross section of a red apple.
After some time, one of the halves of the apple, not smeared with lemon juice, darkened, and the one that was “protected” by lemon juice remained white.
I cut the lemon. I smeared one half of the apple with lemon juice, and placed the second half of the red apple cut side up on a plate. I did the same thing in the same sequence with a green apple. I put both halves of a green apple on a plate, cut side up, and began to observe the changes.
Conclusion. Darkening occurs due to the oxidation of iron, which is contained in apples, by atmospheric oxygen. The acid contained in lemon juice protects the apple cut from oxidation and slows down the oxidation process.
I noticed that the cuts of the red apple did not darken at all, which means that green apples contain more iron and are healthier.
Conclusion
Our life is unthinkable without metals. In our creative work dedicated to iron and aluminum, I expanded my knowledge about these elements, simple substances, metals, their properties and applications.
The role of iron and aluminum in the development and establishment of the technical culture of mankind is exceptionally great. Hardness, ductility, and malleability made them an indispensable material for the manufacture of tools and production. Looking out onto the street, we see hundreds of cars, each of which is made of iron. Cables, bridges, rails, trams, trains, and finally airplanes are made from iron or aluminum alloys. There are metals everywhere!... Well, we ourselves have these metals. They are used to carry out various processes in the body.
In our theoretical part, we characterized the structure and properties of iron and aluminum, their use in medicine, and described what happens when there is an excess or deficiency of ions of these elements.
As a result of the work done, we made the following conclusions:
Iron and aluminum ions have a vital effect on the human body.
Aluminum ions in large quantities are hazardous to health. Therefore, you should only cook in aluminum cookware and not store food. Do not get carried away with taking heartburn pills.
Iron ions have a beneficial effect on the human body. Therefore, it is necessary to eat foods rich in iron. Such as persimmons, apples, walnuts.
List of used literature
- Gorynin I.V. et al. Aluminum alloys. Application of aluminum alloys. Help Guide. Moscow "Metallurgy", 1978
- Hatch J. E. Aluminum. Properties and physical metallurgy. Directory. Moscow, "Metallurgy", 1989
- Rabinovich V. A., Khavin Z. Ya. Brief chemical reference book.
- Brief chemical encyclopedia. "Soviet Encyclopedia", 1963
- Karapetyants M.Kh., Drakin S.I. General and inorganic chemistry. "Chemistry", 1981
- Venetsky S. I. Stories about metals
- Beckert M.. Iron. Facts and legends.