Brass is a double or multi-component copper-based alloy, where the main alloying component is zinc, sometimes with the addition of tin (less than zinc, otherwise you will get traditional tin bronze), nickel, lead, manganese, iron and other elements. According to the metallurgical classification, it does not belong to bronze.
Brass is one of the most popular and widely used materials.
The alloy comes in different shades - from dark yellow to yellow-red, golden, white, and even greenish. What color the alloy will acquire depends on the percentage of additives. Brass is characterized by strength, ductility, good fluidity, corrosion resistance, slight shrinkage, and it also lends itself well to any type of processing.
Brass is a double or multi-component copper-based alloy alloyed with zinc
Brass is a corrosion-resistant alloy of zinc and copper that combines high strength with good machinability.
Copper is the basis of brass, determining the basic properties. Zinc is an alloying component, the percentage of which can reach 49%. The composition may contain other alloying elements, but their mass fraction is usually no more than 1.5%. Brass alloys have a bright yellow color that varies depending on the percentage of the main components. A successful combination of characteristics makes brass the optimal choice for the manufacture of pipes, profiles, plumbing fittings, parts of some mechanisms and other elements for which good corrosion resistance combined with strength is important. A characteristic feature of the production process is that about half of all zinc used comes from recycling plants. The surface of brass products can be easily polished, but darkens over time when exposed to air, so it is often varnished or nickel-plated.
Types of brass
It is customary to distinguish single-phase brass or so-called alpha-type brass, containing up to 30-35% zinc, and two-phase varieties of alpha-beta type with a higher (up to 47-50%) content of the main alloying component than in single-phase brasses. Single-phase brasses are more ductile; with increasing additives, the strength of the brass increases, but its ductility significantly decreases.
Two-phase brass alloys are significantly less ductile than single-phase ones. This change in properties due to a change in composition is explained by the fact that with an increase in the number of alloying additives, the structure of the alloy invariably changes. Moreover, the strength of two-phase brass varieties is significantly higher than that of single-phase ones. Dual-phase brass alloys may contain up to 6% lead as an additional alloying additive.
Brass alloys with a relatively low zinc content of up to 10% are usually called tombacs, and with a zinc content of 10-20% - semi-tompacs.
Chemical composition of brass
Brass is close in chemical composition to bronze, and both brass and bronze are based on copper. A significant difference is that the main alloying component in brass alloys is zinc, the content of which can reach 45%.
Let's take a closer look at the properties of the main components of brass.
Zn (zinc) element of the periodic table, atomic number 30. The element belongs to the secondary subgroup 2 of group IV of the IV period. The metal is transitional; it is characterized by the appearance of electrons in d- and f-orbitals in atoms. The metal has a light blue tint, which darkens in air, becoming covered with an oxide film.
Cu is the main component of the alloy. The element belongs to group 11 of the IV period of the Mendeleev periodic system and has atomic number 29. The metal, like zinc, is transitional. The metal has a beautiful yellowish-golden color. When an oxide film forms, copper acquires a reddish tint.
As discussed above, brass can have a structure that consists of an alpha phase or an alpha-beta phase.
As alloying components, brass may include:
- Mn to increase the strength of alloys, including anti-corrosion. In addition to Mn, additional introduction of Al, Sn, and Fe enhances the strength and anti-corrosion characteristics of the metal.
- Sn to improve salt water resistance. Such brass alloys have acquired a “secret” name - marine brass and are widely used in areas of contact with sea water.
- Ni gives the joint high strength characteristics and also increases anti-corrosion properties.
- Pb is used if the brass part will be cut. This element makes the metal more malleable during machining. Brass alloyed with lead is called automatic brass.
- Si is necessary to enhance the antifriction characteristics of the alloy, which makes it possible to safely use it along with bronze in some technological units, bearings, etc. But it is worth noting that silicon significantly reduces the hardness and strength of brass products.
The table below shows the chemical compositions of some brands of brass alloys. The table shows that all brands have different compositions; the copper content in some brands can reach 91%.
Properties of brass depending on the percentage of components, heating temperature
When the percentage of solid solution components changes or additional alloying elements are introduced, the properties of the resulting metal also change.
Let's try to trace how the properties of the metal change when the Zn content changes:
- When the zinc content is less than or equal to 30%, the hardness and elasticity of the metal increase.
- With a further increase in zinc content, the elasticity begins to decrease due to the compaction of the alpha solution. At the same time, hardness increases.
- But when the zinc content reaches 45%, the hardness also drops.
Due to its elasticity, brass can be easily processed under pressure. This especially applies to single-phase alloys. The temperature regime for changing shape should not fall within the range of 300-700°C; this is the “fragile zone” of the metal. Alpha-beta varieties exhibit increased ductility when heating temperature increases above 700°C.
Thus, the content of chemical elements in a metal directly affects its technological parameters and properties. Alpha-brass alloys are characterized by increased ductility, alpha-beta varieties are durable and strong, but they are not suitable for deformation processing. The brass alloy has increased resistance to corrosion and sea water due to the addition of alloying components, which allows its use in areas of constant exposure to aggressive environments.
Dependence of characteristics on the composition of brass
The properties of brass are directly determined by the mass fractions of the main components. With a zinc content of up to 35%, brass has a single-phase structure (alpha phase), which is characterized by high ductility over a wide temperature range. With a larger proportion of zinc, brass alloys acquire a two-phase structure and become quite brittle under natural temperature conditions.
On sale are two-component grades, consisting only of zinc and copper, and multi-component grades - alloyed with additional chemical elements that modify their properties. Additional alloying components allow you to change individual characteristics, such as strength, toughness, ductility, etc.
- Brass mesh
- Brass square
- Brass sheets L63
- Brass sheets LS59-1
- Brass bands
- Brass rods L63
- Brass wire L63
- Brass pipes L63
- Brass hexagonal rods LS59-1
- Brass hexagonal rods L63
- Brass round bars LS59-1
- Brass wire LS59-1
- Brass pipes L68
Types of brass metal products
The main types of brass metal products are as follows:
- brass rods - long parts with a round, square, rectangular cross-section;
- brass plates - flat pieces 2.5 cm thick or more;
- brass wire for electrical engineering and other industries;
- brass pipe for carrying communication lines;
- brass circles for the manufacture of machine tools, instrument making, etc.;
- brass sheets for various industries, etc.
For each type of brass metal, a certain grade of metal with a strictly regulated chemical composition is required.
Brands of brass
- L63 - poorly processed by mechanical methods, used for the manufacture of nuts, bolts, machine parts and heating engineering elements;
- LS59-1 – well processed, used for the manufacture of nuts, bolts, gears and bushings.
The first grade is a two-component alloy with a mass fraction of zinc up to 37%. In the second, the zinc content reaches 40%, but despite this, it is more ductile and more technologically advanced due to additional alloying with lead.
Manufacturability of brass
Copper- and zinc-based metals lend themselves well to machining, making it possible to turn any parts from blanks and preliminary castings. In addition, they lend themselves well to soldering.
The main disadvantage is the tendency for brass with a high zinc content (more than 20%) to crack, especially when used in a humid environment and in the presence of ammonia vapor. The first sign of a decrease in the strength of rolled brass metal is the loss of natural color; other properties gradually deteriorate.
Composition and classification of brasses
The classic composition assumes the presence of copper and zinc in the alloy in a ratio of 2:1, respectively. The Ancient Romans knew such brass. Skeptics will remember that zinc in its pure form was discovered in the 16th century. But in the case of Ancient Rome, we are talking about zinc-containing rock, which at that time was already being processed.
At that time, it was believed that it was the presence of zinc that determined the color, and only later did it become known that the sunny shade of the brass alloy is obtained due to the fact that the presence of zinc dilutes the copper redness.
- Brass is divided into two-component (simple) and multi-component (special).
One of the markings for products made from brass indicates the percentage of components. So the letter L indicates the type of alloy - brass. and the adjacent numerical index indicates the copper content in the composition. For example, “L80” stands for “brass consisting of 80% copper and 20% zinc.”
Two components are not a mandatory requirement. If there are more of them, then each component introduced into the brass composition is displayed in the marking using the corresponding letter symbol following the letter L. Tin, nickel or lead can be used as additives. At the same time, brass changes its properties.
Additives are introduced into the alloy to achieve certain purposes. For example, brass in classical proportions cannot be used in shipbuilding. All thanks to the instability of brass to the effects of saline solutions (sea water). Additives introduced into the alloy solve this problem while maintaining the basic characteristics.
- According to the degree of processing, alloys are divided into: wrought (brass strip, wire, pipe, brass sheet) and cast (fittings, bearings, instrument parts).
Wrought two-component brass
Deformable multi-component brasses
Foundry brasses
Ways to improve the characteristics of brass alloys
The fragility of the alloy can be significantly reduced by annealing, carried out in the temperature range of 240-260 °C. During heat treatment, the strength properties of the material are improved and residual stress is eliminated. The main way to influence performance characteristics (strength, density, ductility, color, etc.) is the introduction of alloying components.
A pure alloy of zinc and copper is called two-component; if alloying elements are present in the composition, it is called multi-component. The most common alloying additives are lead, silicon, nickel, iron, tin and manganese. Their percentage is usually small (up to 1-1.5%), but the characteristics change dramatically. If you exceed the norm, the quality of brass metal products can significantly deteriorate.
The introduction of silicon and lead makes it possible to improve the strength and antifriction characteristics of brass, due to which the wear resistance of mechanical parts made from it significantly increases. If the mass fraction of silicon exceeds technical standards, the characteristics of brass may deteriorate sharply. Also, lead and silicon, when proportioned, can improve the aesthetic properties of the material.
Tin, aluminum and manganese increase tensile adaptability, and the addition of iron and manganese increases the elongation rate. It is important to note here that all other alloying additives have a negative effect on the elongation index.
To increase the anti-corrosion properties, nickel, tin, manganese and aluminum are added to brass alloys. The addition of nickel helps prevent cracking in high humidity conditions. An additional positive effect of tin alloying is to increase strength, density and resistance to sea water and salt spray. Therefore, such materials are used in devices intended for shipping.
Alloying with lead increases ductility and manufacturability, making brass easier to machine cut. When processed on a lathe, the workpieces do not crack. The chips are small and the surface is almost perfectly smooth, so the finished part does not require finishing.
Arsenic is rarely used as an alloying component for zinc and copper alloys. Typically, parts alloyed with it are used to work in aggressive chemical environments. If iron and nickel are added to the alloy simultaneously with arsenic, the durability of the finished product increases significantly, and it can work in contact with weak solutions of alkalis and acids.
Characteristics
There are no uniform basic parameters for brass alloy. However, the material can be easily processed by pressing and mechanical action, with good corrosion resistance.
| Thermal conductivity | 121 W/(m K) |
| Density | 8921 kg/m³ and 7140 kg/m³ |
| Melting temperature | 932 °C |
| Crystal system | cubic system |
| Young's modulus | 115 ± 20 GPa, 100 GPa and 130 GPa |
| Poisson's ratio | 0,37 |
| Young's modulus under compression | 50 GPa |
Peculiarities
There is no single value for the density, melting point, threshold of thermal and electrical conductivity of brass. The parameters are determined by the number and proportion of elements.
The “richer” the composition, the more variations:
- Compared to bronze, brass wears out faster and is less durable. They conduct current and heat worse.
- Not so resistant to corrosion and aggressive environments (sea water, dissolved organic acids). Although on this basis they are superior to pure copper.
- When exposed to acid, brass turns pale to discolored. At the point of contact, a drop of aggressive solution bubbles.
Based on this chemical property, it can be easily distinguished from gold. Nothing happens to him.
- In the range of 212-624°C, the structure of brass is destroyed: the material crumbles.
The rate of corrosion increases with increasing temperature. This phenomenon is neutralized by the final stage of processing: the products are fired at low temperatures.
The influence of alloys on the properties of the alloy
The shortcomings of the material are smoothed out by adding ligatures. Along the way, alloying elements enhance the advantages:
- Tin, nickel, and manganese significantly increase the threshold of strength and corrosion resistance of the alloy.
- Silicon reduces strength and hardness. However, it is easier for a welder to work with such material.
- Lead also reduces utilitarian performance but makes cutting easier.
- Aluminum creates a protective oxide film on the melt, inhibiting the “flight away” of zinc during melting.
The influence of alloys is reflected in the unofficial names of metal alloys. Thus, tin brass is called marine brass. Lead - automatic, since processing is carried out on automatic machines.
Lead alloys are the most popular among brasses.
Silicon-lead material has low abrasion. Valued as a backup for expensive bronzes.
Casting brass alloys
There are two main types of brass alloys for mass consumption: casting and wrought (jewelry is also included in a separate group). The characteristics and processing technologies of casting brasses are described in GOST 17711. Materials of this type are characterized by increased density, reduced gas content and good corrosion resistance. Due to the partial evaporation of zinc during the casting process, the metal is well deoxidized, but it is important to control this process so that the characteristics of the finished product correspond to the calculated values.
Cast brasses are characterized by reduced segregation (heterogeneity that occurs during the casting and crystallization process), increased melt fluidity and a low shrinkage coefficient. In terms of mechanical characteristics, finished parts made from this metal are similar to products made from aluminum and tin bronze, while their cost is significantly lower due to a simpler production technology.
Of course, cast brass alloys also have certain disadvantages. Thus, during crystallization, quite large cavities can form on the surface of products, leading to a significant percentage of defects. It is also important to consider that due to the evaporation of zinc, smelting must be carried out using special fluxes.
Application of brass
Brass is a universal material, therefore it is widely used in many areas. The material, which is highly resistant to corrosion, is actively used in mechanical engineering and shipbuilding. It also serves as a material for the manufacture of vessels, fasteners, squares for decorating books, pectoral crosses and military insignia: orders and medals. Brass is in demand in the production of pipes, taps, couplings, fittings, and other parts that are in demand in plumbing. Even when creating jewelry, brass is used.
Wrought brass alloys
This category of zinc and copper alloys is pressure processed. The characteristics and technology of working with them are regulated by the GOST standard 15527. They are supplied in the form of rolled metal and blanks for subsequent processing and production of parts of the required shape. Additionally, there are two categories of copper-zinc alloys: double (two-component) and special (multicomponent). The two most popular grades of wrought alloys are L63 (two-component) and LS59-1 (multicomponent, alloyed with lead).
Based on their structure, single-phase and two-phase alloys are also distinguished. Single-phase brass has a uniform, unchangeable color and has good processability. Two-phase ones have increased density, they become more fragile and are less susceptible to cold processing. The melting point for all copper-containing alloys is approximately in the same range.
What types of rolled products are made from brass?
Due to the high ductility of deformable brass, the range of rolled products is very wide. You can buy rolled brass at METAL BUREAU at minimal prices in the form of forgings, sheets, strips, strips, profiles, pipes and wire. The range of each of these types of rolled products is regulated by its own standard. In particular, sheets and strips - GOST 931-90, strips - GOST 2208-91, rods - GOST 2060-90, pipes GOST 494-90, wire - GOST 1066-90. At the same time, the chemical composition of all pressure-processed brasses is subject to GOST 15527-2004.
Physico-chemical properties of brass alloys
In appearance, brass resembles bronze, which is why they are confused or even identified. But in bronze the main alloying component is tin, not zinc, so these are two completely different copper-containing metals with significantly different physicochemical properties.
Zinc (Zn, Zincum) is in position 30 in the periodic table. He is included in the secondary subgroup of the second group of the fourth period. Under normal conditions, pure zinc is a brittle metal with a characteristic bluish tint. In air it quickly oxidizes, and if a zinc stick is bent, a characteristic cracking sound is heard (in this way zinc resembles tin). Pure zinc does not occur in nature.
Copper (Cu, Cuprum) is located right in front of zinc in the periodic table - in 29th position. It belongs to the elements of the eleventh group of the fourth period. In its pure form, it is a soft, ductile metal of rose-gold color. Under natural conditions, the surface of purified copper quickly oxidizes, combining with atmospheric oxygen. Despite this, it is found in its native form, thanks to which it became one of the first metals known to man. The most ancient copper products found during excavations in the village of Catal Huyuk (Turkey) date back to 7500 BC.
Receiving technology
Brass does not occur in nature.
The source material for its production is three types of raw materials:
- Primary. Copper, zinc and other ores are mined from natural deposits.
- Copper, zinc, and other metal scrap suitable for recycling (recyclables). Accumulated at collection points.
- Waste from the metallurgical plants' own production cycle.
Traditional production methods involve the use of furnaces for smelting copper and its alloys. Typically these are electro-induction units equipped with a magnetic core and operating at low frequencies.
Microstructure of ground and etched brass alloy under 400x magnification
Alloy production process:
- Hot copper is placed in a furnace.
- Next, lump zinc is loaded.
- Melting takes place at 875-945°C.
- Alloying additives are added to special brasses.
- The mass is mixed until smooth and poured into molds.
The output is flat or round brass ingots. The smelted products have different hardness, degree of hardening and aging.
Advanced technologies include the installation of ventilation for exhaust during melting of vapors that are dangerous to humans.
Influence of the zinc proportion on the properties of brass alloy
The basic properties of the combination of zinc and copper depend on the percentage of the main components. Since pure copper is ductile, alloys with less than 30 percent zinc also have this property. An increase in the proportion of zinc gradually makes the metal more brittle, and with the appearance of the β'-phase, the brittleness increases sharply. In this case, the hardness increases up to 45 percent zinc content, after which this parameter sharply decreases.
Since one of the main forms of brass parts is pressure deformation, it is important to consider the ductility of the alloys used. Single-phase compositions retain plasticity and can be stamped at ordinary temperatures, but in the range of 300-700 °C they can acquire undesirable brittleness. Two-phase alloys acquire the ductility necessary for stamping only at temperatures exceeding 700 °C.
Melt brass technology
To obtain the melt, two main technologies are used:
- melting in crucibles made of refractory clay by heating in a flame or shaft furnace;
- melting in a reverberatory furnace without the use of crucibles.
Molten metal is poured into sand molds to produce blanks and ingots. It is important to consider that part of the zinc evaporates during the process, so it is necessary to choose an alloy in which its proportion will be slightly higher. The correction for evaporation is calculated individually for a specific technology so that the proportions of metals in the finished product correspond as much as possible to the design values.
Brass marking
To avoid confusion, the first letter in the marking of copper-zinc alloys is always “L”. If the alloy is two-component, then the marking consists only of this letter and two numbers indicating the percentage of copper. Thus, the marking of one of the most common alloys L63 implies 63% copper and up to 37% zinc (acceptable values are 62-65% for copper and 34-37.5 for zinc, the amount of other impurities is no more than 0.5%).
The addition of additional alloying components in significant quantities is also reflected in the designation of the alloy grade. The name of the main alloying component is also added to the name. For example, the popular brand LS59-1 is deciphered as follows:
- L – brass;
- C – lead;
- 59 – percentage of copper;
- 1 – lead content.
Decoding brass grades with a large number of components is done in a similar way. The letters after “L” indicate additional alloying impurities, and a hyphen (or several hyphens) indicates their weight percentages. For example, the marking LAZhMts70-5-3-1 implies the presence of 5% aluminum, 3% iron and 1% manganese. The share of zinc is respectively 20-21% (including 0.5-0.75% impurities).
Let's analyze the chemical properties of brass and the influence of all its components.
The designation of all varieties of brass according to GOST is the capital L. For a 2-component alloy, it is followed by the percentage of copper, and the remainder is zinc. An example is L63. For multi-component brass, the first letters of alloying elements are added to L, and their percentage in the composition is added to the number separated by a dash (LS59-1). The amount of zinc is calculated by subtracting the sum of these numbers from 100.
Zinc improves the mechanical properties of brass, increasing its ductility and strength. Its maximum ductility is achieved at 30 percent zinc content. If Zn is more than 39%, the ductility deteriorates, and the strength of the alloy, on the contrary, increases sharply.
Arsenic prevents dezincification of brass when it is in aggressive fresh water at room or elevated temperatures.
Lead enhances the antifriction properties of brass and promotes better machinability of workpieces by cutting. This element acts as a lubricant and reduces wear on cutting tools. The chips become brittle, brittle and do not stick to the cutters or cutters. The surface of the parts is smooth, with the required roughness parameters.
Iron reduces the grain size of brass, increases the hardness of the alloy and increases its recrystallization temperature.
Tin, aluminum and nickel make brass more resistant to corrosion in seawater and air while increasing its strength.
Brass is a copper-zinc alloy. Its components in the presence of an electrolyte solution (water, etc.) form a galvanic couple. Moreover, the more active metal, zinc, is destroyed. The brass dezincification process starts. This is corrosive destruction, accompanied by darkening of the surface of the material and the release of white zinc oxides on it.
Because of this, the use of brass fasteners with mating elements made of steel, bronze or aluminum alloys is not allowed. Those. brass parts can only be connected to each other or using chemically inert plastics (polyamide, etc.). In addition, brass must be stored in warehouses at positive temperatures without significant changes. High air humidity and condensation are not allowed.
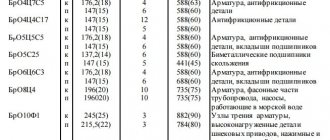
| Conformity table of domestic and foreign brands of brass | |||
| ASTM | DIN | GOST | |
| C21000 | CuZn5 | 2.0220 | L96 |
| C22000 | CuZn10 | 2.0230 | L90 |
| C23000 | CuZn15 | 2.0240 | L85 |
| C24000 | CuZn20 | 2.0250 | L80 |
| C26000 | CuZn30 | 2.0265 | L70 |
| C26200 | CuZn33 | 2.0280 | L68 |
| C26800 | CuZn37 | 2.0323 | L63 |
| C27200 | |||
| CuZn40 | L60 | ||
| C35000 | CuZn38Pb5 | 2.0371 | LS59-1 |
| C37100 | |||
| C35600 | CuZn36Pb3 | 2.0375 | LS63-3 |
| C36000 | |||
| C68700 | CuZn20A12 | 2.0460 | LAMsh 77-2-0.05 |
| C37700 | CuZn39Pb2 | 2.0380 | LS59-2 |
| C38500 | CuZn39Pb3 | 2.0401 | LS59-3 |
| C37800 | CuZn30Pb2 | 2.0402 | LS58-2 |
| CuZn20Al2 | LA77-2 | ||
| CuZn38AlIMn2Al1 | LAZ60-1-1 | ||
| CuZn40Mn1 | LMts58-2 | ||
| CuZn40Al1Mn | LMtsA57-3-1 | ||
| CuZn28Sn1 | LO701 | ||
| CuZn38Sn1 | LO62-1 | ||
| CuZn35Pb2 | LS63-2 | ||
| CuZn38Pb2 | LS60-2 | ||
The density of brass, as one of its physical properties, differs little from copper. If the permissible amount of silicon in the alloy is exceeded, the brass will have less density and strength.
Additives to the alloy of manganese, tin and aluminum contribute to an increase in the tensile strength of brass parts. To increase the elongation factor, alloying with iron is necessary.
Due to the significant copper content and the presence of zinc, the electrical properties of brass are excellent. Electrodes for erosion machines are made from it.
The melting points of brass are in the range of 855 – 965 for lead alloys and 885 – 1070 °C for two-component alloys. This value decreases with increasing zinc content.
| Technical comparison of Copper and brass grades L63 and LS59-1 | |||
| Fur. show | Copper | L63 | LS59-1 |
| Electrical resistivity | 0,018 | 0,065 | 0,065 |
| Thermal conductivity | 0,925 | 0,25 | 0,25 |
| Impact strength | 17.0 | 14.0 | 5.0 |
| Tensile strength, shear in MPA | 210 | 240 | 260 |
| Machinability | 18% | 40% | 80% |
Brasses subjected to processing by plastic deformation (rolling, bending, pressing, forging, embossing) are single-phase (L63, up to 30% zinc) or two-phase (40 - 45% Zn). The last category (for example, LS59-1) is excellently processed only in a hot state, and 1-phase - in a cold or heated state. In order to relieve residual stresses inside the part and increase its hardness and strength, products after stamping and similar processing are subjected to heat treatment (hardening) at a temperature of 240 - 260 oC.
Brass is similar in color to bronze. But the latter is attracted to a magnet, while brass is practically not. In addition, brass has a high density and, accordingly, a workpiece made from it with the same configuration is heavier than bronze. When brass parts are heated to 600 - 650 °C (dark red tint), ashen zinc oxide appears on their surface, which bronze does not have. Brass is more ductile at the expense of wear resistance and strength, which are higher in bronze. And bronze resists corrosion better when in contact with salty sea water. Under these conditions, only tin brass of the LO brand is comparable to it. The brass at the fracture site is light with a fine-grained structure, bronze is painted dark brown and has large grains.
The advantages of brass parts are:
- Resistance to corrosion destruction, which manifests itself when products are exposed to air, sea water, most organic and carbon dioxide. Brass is more resistant to oxidation processes than stainless steel.
- Pliability under pressure is characteristic of alloys that do not contain lead. This facilitates their forging and stamping (with or without heating).
- Low thermal conductivity, so when the temperature drops, the properties of the products do not change.
- There is no residual magnetization at the end of exposure to current or electromagnetic field. Therefore, such parts (for example, made from L63A material) do not create interference and are used in devices with increased sensitivity to interference.
Brasses are resistant in the following environments (at normal temperatures):
- air, incl. nautical
- dry steam at low speeds (oxygen, carbon dioxide and ammonia accelerate corrosion)
- fresh water (ammonia, hydrogen sulfide, chlorides, acids accelerate corrosion)
- in sea water at low water speeds
- dry halogen gases
- antifreezes, alcohols, freons
Relatively stable:
- alkali without stirring
Brasses are unstable in the following environments:
- wet saturated steam at high speeds
- mine waters
- oxidizing solutions, chlorides
- mineral acids
- hydrogen sulfide
- fatty acid
The main areas of application of brass products are the chemical, aviation and shipbuilding industries, refrigeration engineering, electrical engineering and energy (contacts, busbars, power transformers), production of high-precision electronic devices and sensors. Brass is used in everyday life for decorating the interior of premises, installing plumbing fixtures and making furniture, assembling acoustic musical instruments, wooden structures in construction, for making information and advertising signs, and souvenirs.
The main brands of brass that are widely used are:
- Two-component (L63, L68, L70, etc.).
- Multicomponent - aluminum (LA77-2, LA 85-0.5, etc.), silicon (LK80-3), manganese (LMts58-2), tin (LOK59-1-0.3, LO60-1), nickel (LN65-5) and lead (LS59-1, LS63-3).
The brass used for the production of fasteners includes the LS59-1 grade. This multicomponent alloy contains up to 60% copper; 42.2% zinc; 1.9% lead and 0.75% impurities. The melting point is 885...895 oC. This type of brass can be easily processed by pressure, but it is mainly used for making parts by cutting. Lead will facilitate drilling, milling and turning of workpieces.
The range of products made from LS59-1 includes:
- Rods of various sections, wire and rolled profiles.
- Strip, plate, sheet and round pipe.
The delivery state of the blanks is soft, semi-hard and hard.
This brass has excellent wear resistance. Therefore, guides for machine tools and sleeves for sliding bearings are made from it. But due to its increased fragility, such material is not resistant to impacts and cannot be used for load-bearing elements.
For the manufacture of decorative furniture fittings, rivets and other fasteners, two-component brass L63 (Cu – 65%, Zn – 38%) with a melting point of up to 910 °C is used.
It is used to produce sheet metal, wire, round pipes and various rods. Thanks to the single-phase structure of the crystal lattice, this alloy is more ductile than other brasses and is excellently deformable using all known technologies (drawing, drawing, stamping, etc.). To maintain decent corrosion resistance, L63 parts produced by cutting are pre-annealed. Such brass products do not oxidize in the environment of liquid and gaseous refrigerants, other halogen-containing substances, aqueous solutions of antifreeze and alcohol, and dry water vapor.