How to determine the density of a metal
Density is an important physical quantity for any state of matter. In this article we will consider the question of what is the density of metals, we will provide a table of this parameter for chemical elements and we will talk about the densest metal on Earth.
What physical characteristics are we talking about?
Density is a quantity that characterizes the amount of a substance present in a known volume. According to this definition, it can be calculated mathematically as follows:
ρ = m/V.
This quantity is denoted by the Greek letter ρ (rho).
Density is a universal characteristic because it can be used to compare different materials. This fact can be used to identify them, which is what the Greek philosopher Archimedes did, according to legend (he was able to identify the fake gold crown by measuring the value of ρ for it).
This parameter for a specific material depends on two main factors:
- on the mass of the atoms and molecules that make up the substance;
- on average interatomic and intermolecular distances.
For example, any of the transition metals (gold, iron, vanadium, tungsten) has a higher density than any carbon material, since the mass of an atom of the latter is tens of times less. Another example. Graphite and diamond are two carbon structures. The second one is more dense because the interatomic distances in its lattice are smaller.
Density of metals
This is the most numerous group of the periodic table. A metal is any substance that has high thermal and electrical conductivity, a characteristic surface shine when polished, and the ability to undergo plastic deformation.
This chemical element has low electronegativity compared to substances such as nitrogen, oxygen and carbon. This fact leads to the fact that in bulk structures metal atoms form metallic bonds with each other. It represents the electrical interaction between positively charged ionic bases and a negative electron gas.
Metal atoms are arranged in space in an ordered structure called a crystal lattice. There are only three types:
- cubic;
- BCC (body-centered cubic);
- HPU (hexagonal close-packed);
- FCC (face centered cubic).
The density of metals is a physical quantity that depends on the type of crystal lattice. Below is a table of this parameter for all chemical elements in g/cm3, which under normal conditions are in a solid state.
It follows from the table that the density of metals is a value that varies over a wide range. Thus, the weakest is lithium, which, with the same volumes, is two times lighter than water. The density of the rare metal osmium is the highest in nature. It is 22.59 g/cm3.
The density of metals is a characteristic that can be determined in two fundamentally different ways:
- experimental;
- theoretical.
Experimental methods are of the following types:
- Direct measurements of body weight and volume. The latter is easy to calculate if the geometric parameters of the body are known and its shape is ideal, for example, a prism, pyramid or ball.
- Hydrostatic measurements. In this case, special scales are used, invented by Galileo in the 16th century. The principle of their operation is quite simple: first, a body of unknown density is weighed in air, and then in liquid (water). After this, the required value is calculated using a simple formula.
As for the theoretical method of determining the density of metals, this is a fairly simple method that requires knowledge of the type of crystal lattice, the interatomic distance in it and the mass of the atom. Next, using the example of osmium, we will show how this method is used.
Density of the rare metal osmium
It is found in small quantities on our planet. Most often it is found in the form of alloys with iridium and platinum, as well as in the form of oxides. Osmium has an HCP lattice with parameters a = 2.7343 and c = 4.32 angstroms. The average mass of one atom is m = 190.23 amu.
The above figures are sufficient to determine the value of ρ. To do this, you should use the original formula for density and take into account that one hexagonal prism contains six atoms. As a result, we arrive at the working formula:
ρ = 4*m/(√3*a2*c).
Substituting the numbers written above and taking into account their dimensions, we arrive at the result: ρ = 22,579 kg/m3.
Thus, the density of the rare metal is 22.58 g/cm3, which is equal to the experimentally measured tabular value.
Density and specific gravity of metals and their alloys
Metal products are used in all spheres of human activity. Metals in the scientific sense are simple substances with specific properties (metallic luster, malleability, high electrical conductivity). In everyday life and in production, their alloys with other elements are often used. These solidified melts are also commonly called metals.
Definition and use of density
As you know, to find the density of a substance, its mass is divided by its volume. Density is a physical and chemical characteristic of a substance. She is constant. Materials for industrial production must meet this indicator. To denote it, it is customary to use the Greek letter ρ.
The density of iron is 7874 kg/m³, nickel - 8910 kg/m³, chromium - 7190 kg/m³, tungsten - 19250 kg/m³. Of course, this applies to hard alloys. In the molten state, substances have different characteristics.
In nature, only some metals are present in large quantities. The specific gravity of iron in the earth's crust is 4.6%, aluminum - 8.9%, magnesium - 2.1%, titanium - 0.63%. Metals are indispensable in most areas of human activity. Their production is growing year by year. For convenience, metals are divided into groups.
Iron and its alloys
Ferrous metals are usually called steel and cast iron of various grades. An alloy of iron and carbon is considered steel if the iron content is at least 45% and the carbon content is 0.1%-2.14%. Cast iron, accordingly, contains more carbon.
To obtain the necessary properties of steels and alloys, they are alloyed (alloying additives are added during remelting). This is how the specified grades are melted. All metal grades strictly comply with certain technical conditions. The properties of each brand are regulated by state standards.
Depending on the composition, the density of steel varies in the range of 7.6–8.8 (g/cm³) in the SGS or 7600–8800 (kg/m³) in the SI (this can be seen from Table 1).
Of course, steel has a complex structure; it is not a mixture of different substances. However, the presence of these substances and their compounds changes properties, in particular density.
Therefore, high-speed steels with a high tungsten content have the highest densities.
Non-ferrous metals and their alloys
Products made of bronze, brass, copper, aluminum are widely used in production:
- Bronzes are usually alloys of copper with tin, aluminum, lead and beryllium. However, in the Bronze Age, when the proportion of bronze in the total mass of metal products was almost 100%, these were copper-arsenic alloys.
- Zinc-based alloys - brass. Brass may contain tin, but its amount is less than zinc. Lead is sometimes added to produce free-flowing chips. In addition to jewelry alloys of brass and bronze, they are needed for machine and marine parts, hardware, and springs. Some varieties are used in aviation and rocketry.
- Duralumin (duralumin) - an alloy of aluminum and copper (copper 4.4%) is a high-strength alloy. Mainly used in aviation.
- Titanium is stronger than many steel grades. At the same time it is twice as light. These qualities have made it indispensable in most industries. It is also widely used in medicine (prosthetics). The share of titanium in the production of aircraft reaches 70% of all smelted in the world. About 15% of titanium is used for chemical engineering.
- Silver and gold are the first metals with which man became acquainted. Throughout the history of mankind, these metals have mostly been used for jewelry. And currently the trend continues.
- Due to its high refractoriness, tungsten is indispensable in instrument making. Its high density allows it to be used as radiation protection.
- Nickel and chromium form nichrome - a heat-resistant plastic alloy, very durable and reliable.
Different grades of steel and cast iron, bronze and other metals have different chemical compositions and different densities. The densities of all required materials are measured and systematized. Tables containing this data are available to users. With their help, you can easily find the mass of a product of a given shape.
Transportation of metal products
In the cargo transportation system, such a concept as “volumetric weight” is involved. If the mass of an object in one cubic meter is 167 kg, then this weight is considered physical, and if it is less, it is considered volumetric. For example, the mass of a cube of carbon steel is 7750 kg. In other words, the volumetric weight of steel is 7750 kg. These calculations are needed to determine how much volume the transported cargo will occupy.
However, depending on what metal products are transported, the volume will vary. Let's assume that there are several different hardware of the same grade of steel. In theory, they have the same density. However, ingots, large-grade products and coils of wire have different volumes, and therefore, when transported, they will take up more or less space in transport. Thus, they have different volumetric weights. Under any conditions, a cubic meter of steel is more than 167 kg, therefore, it cannot be called volumetric.
- Author: admin
Rate this article:
- 5
- 4
- 3
- 2
- 1
(1 vote, average: 5 out of 5)
Share with your friends!
Density of steel
The first mentions of steel are contained in Indian sources dating back to approximately 1 millennium BC. e. Steel swords made by Indian craftsmen were stronger and sharper than bronze ones. Steel was processed in the Middle East and Ancient Rome. It was steel swords and armor that helped the Roman legions in their victorious march through the ancient world.
The rebirth of the material occurred in the 19th century, when the open-hearth method of its smelting was developed, which made it possible to obtain alloys of high and stable quality in large quantities. In the 20th century, steel became the main structural material. One of the important characteristics of any material is its density - the mass of a substance per unit volume.
Density of steel
Density is measured in grams per cubic centimeter or tons per cubic meter. The digital density value for these two units of measurement will be the same. The density of the same material at different temperatures changes due to the phenomenon of thermal and volumetric expansion. For most substances, including metals, density decreases with increasing temperature.
Density of structural alloy steel
Structural alloy alloys are used in the production of highly loaded critical structures, including those operating in aggressive environments. The density of grade 30KhGSA is close to the standard value of 7.85 t/m3, the density of low-alloy structural steel for welded structures
Low-alloy alloys have excellent weldability and high corrosion resistance, so they are widely used for critical structures in construction and shipbuilding. The HC of steel in this group ranges from 7.85-7.87 t/m3 and is given in the table:
| Group | Brand | Density |
| low alloy structural | 09G2S | 7,85 |
| high carbon | 70 (VS and OVS) | 7,85 |
| medium carbon | 45 | 7,85 |
| low carbon | 10, 10A, 20, 20A | 7,85 |
| carbon structural | St3sp, St3ps | 7,87 |
Density of structural steel with increased machinability
The specific gravity of 30KhGSA steel used for shafts, axles, and levers is 7.85 t/m3. When heated to 200 ºС it decreases to 7.8. The density of structural bearing steel grade 35ХГ2 is 7.8 t/m3.
The specific gravity of steel 12Х2Н4А, used to create highly loaded gears, piston pins, etc., is 7.84 t/m3 at 20 ºС and decreases to 7.63 when heated to 600 ºС
Density of structural spring steel
Spring-spring alloys have increased elasticity while maintaining high strength and are used for the manufacture of elastic elements of mechanisms - springs, springs, shock absorbers. The density of grade 65G is 7.85 t/m3.
Density of structural carbon quality steel
High-quality structural carbon steel grades 10, 20, 30, 40 has a density of 7.85 t/m3
Density of stainless steel
The density of a substance is calculated by dividing the mass of an object by its volume. Such calculations have already been made for all substances known to man, and metrological services periodically repeat and refine these measurements. In practice, people face another practical task: knowing the material from which the product is made, determine its mass.
The density of a substance is also called specific gravity (or, in everyday life, specific gravity) - that is, the mass of a solid physical body made of a given substance and having a unit volume.
Stainless steel
It should be noted that when using the term “mass”, in 99% of cases people are dealing with weight - the force of attraction of the physical body to the Earth.
The fact is that to determine body weight in a strict physical sense, sophisticated equipment is required, available only in the largest scientific centers.
For practical use, in most cases, conventional, more or less accurate scales using the Earth's gravity and springs, or levers and standard weights, or piezoelements are sufficient.
In practice, to calculate the weight of a linear or square meter of rolled metal, the specific gravity, or density of the material from which it is made, is used. In reference books on the assortment of rolled metal, among the main characteristics of each grade, the mass of a linear or square meter and the density value used in the calculations must be indicated.
In most cases, calculations based on the mass of a linear or square meter are sufficient for practical applications. Raw materials and components are purchased with a certain standardized stock, and before shipment to the consumer, the product is weighed on scales for accurate settlements between contractors.
However, you need to understand that the data in the directory is calculated based on the standard density of steel, most often it is 7.85 t/m3. At the same time, the actual density of a particular steel grade depends on the composition and specific amount of additives and can range from 7.6 to 8.8 t/m3.
This can give an error of up to 10% up or down for a product made from a very light or, conversely, very heavy alloy. For a small amount of metal the difference will be small and can be neglected. However, for complex products that use large volumes of metal, more accurate calculations will be required.
The mass will be needed when creating an application for the purchase of metal. Based on the density of a given alloy, an adjustment is made to the reference values of the mass of one linear or square meter, and then the already specified value is used in the calculations.
How to calculate P or perform 1 meter mass adjustment?
The practical method for determining density is quite simple and is known to us from a school physics course. A sample of material is lowered into a measuring container filled with water to a certain level. The water level rises to a certain height. The volume of displaced water is equal to the volume of the sample. The mass of the sample is determined by weighing on an accurate balance. Density will be equal to the ratio of mass and volume.
To adjust the mass of a linear or square meter, you need to divide the value from the reference book by the density from the reference book and multiply the result by the measured density of the sample material. The corrected value will be obtained.
If similar calculations are expected to be repeated, then it will be more convenient to calculate a correction factor equal to the ratio of the standard density and the density of the sample, and then apply it in the calculations.
Density of 12Х18Н10Т and some stainless steels
Grade 12×18N10T is one of the most widely used stainless steels. The density for it and several popular brands in production is given in the table, the brands are arranged in order of increasing density. The third column shows the density adjustment factor relative to the standard value of 7.85:
| steel grade | Density t/m3 | Correction factor |
| 08Х22Н6Т15Х28 | 7,60 | 0,97 |
| 08Х1312Х17 | 7,70 | 0,98 |
| 04Х18Н1008Х18Н12Б12Х18Н10Т17Х18Н9 | 7,90 | 1,01 |
| 08Х18Н12Т10Х23Н18 | 7,95 | 1,01 |
| 06ХН28МДТ08ХН28МДТ | 7,96 | 1,01 |
| 10Х17Н13М2Т | 8,00 | 1,02 |
| 08Х17Н15М3Т | 8,10 | 1,03 |
Density of other steels and alloys
The specific gravity of steel of other groups is given in the table:
| Steel type | Brand | Density |
| cryogenic stainless structural | 12Х18Н10Т | 7,9 |
| heat-resistant stainless steel corrosion-resistant | 08Х18Н10Т | 7,9 |
| stamped instrumental | X12MF | 7,7 |
| stamped instrumental | 5ХНМ | 7,8 |
| low-carbon electrical (Armco) | A and E; EA; EAA | 7,8 |
| chromium | 15ХА | 7,74 |
| chrome-aluminium-molybdenum nitrided | 38ХМУА | 7,71 |
| chromium-manganese-silicon | 25ХГСА | 7,85 |
| chrome vanadium | 30ХГСА; 20ХН3А | 7,85 |
Steel - concept and its characteristics
Steel is the most common material for the manufacture of structures, parts, mechanisms and tools.
Steels include all alloys of iron and carbon, and the share of iron must be at least 45%, and the share of carbon - less than 2.14 percent.
Carbon, lining up in the molecular structures of iron, increases strength and hardness, but makes the alloy less ductile and malleable. In addition to carbon, the alloy contains metals and non-metals.
The most important characteristics of the alloy include:
- shear modulus;
- elastic modulus;
- density;
- linear expansion coefficient.
Different areas of application of materials require them to have different physical and chemical properties. For example, steel alloys with a high modulus of elasticity are used for the production of springs and spring-type shock absorbers. These properties are purposefully changed as a result of the addition of various additives.
Melting steel
The density of steel, or HC steel, is one of the most important characteristics of the alloy. Based on it, the designer calculates the weight of the part and the total weight of the product, logistics organizes the purchase and delivery of raw materials, blanks and finished products, economists determine the cost. The weight of steel is determined as the product of density and volume.
Steel classification
Depending on the proportion of non-metallic impurities determined by the method of smelting a given grade, steel alloys are divided into:
- especially high quality;
- high quality;
- ordinary quality.
Based on their chemical composition, alloys are also divided into alloyed and carbon.
Carbon steels
Used primarily for the production of welded structures and contains from 0.25 to 2.14 percent carbon. Within the group, they are further divided into subgroups, and also according to the percentage of carbon:
- high carbon (0.6-2.14);
- medium carbon (0.3-0.55);
- low carbon (below 0.25).
They also contain silicon and manganese as additives. In addition to useful, purposefully introduced additives, the alloy may also contain harmful impurities that negatively affect its physicochemical properties:
- phosphorus reduces ductility when heated and increases brittleness when cooled;
- sulfur leads to the formation of microcracks.
Low carbon steel
Other impurities may also be present in the alloy.
Alloy steel
In order for the alloy to acquire the required properties during melting, useful additives or alloying elements are added to it, most often metals such as aluminum, molybdenum, chromium, manganese, nickel, vanadium and others.
The properties of the alloy change quite significantly: the alloy acquires resistance to corrosion, special strength, high malleability, increased or decreased electrical conductivity, etc. An alloy with such additives is called alloy steel.
Based on the percentage of alloying additives, they are divided into three groups:
- highly alloyed – over 11;
- medium alloyed – from 4 to 11;
- low alloy – less than 4.
By area of application, steel alloys are divided into:
- tool - high-strength alloys are used for the manufacture of tools, dies, cutters, drills and cutters;
- structural - used for the production of bodies and components of vehicles, machine tools, building structures;
- special. This group includes alloys with increased resistance to acidic and alkaline environments, radiation, stainless alloys, electrical materials, etc.
Alloy steel
Some additives and treatments increase the density of the material, while others reduce it, for example:
| Processing method or additive | Density change |
| carbon | is decreasing |
| chrome, aluminum, manganese | is decreasing |
| cobalt, tungsten, copper | growing |
| drawing | grows within three percent |
, please select a piece of text and press Ctrl+Enter.
How to determine the density of a metal - Metalist's Handbook
The first mentions of steel are contained in Indian sources dating back to approximately 1 millennium BC. e.
Steel swords made by Indian craftsmen were stronger and sharper than bronze ones. Steel was processed in the Middle East and Ancient Rome.
It was steel swords and armor that helped the Roman legions in their victorious march through the ancient world.
The rebirth of the material occurred in the 19th century, when the open-hearth method of its smelting was developed, which made it possible to obtain alloys of high and stable quality in large quantities. In the 20th century, steel became the main structural material. One of the important characteristics of any material is its density - the mass of a substance per unit volume.
Density of steel
Density is measured in grams per cubic centimeter or tons per cubic meter. The digital density value for these two units of measurement will be the same.
The density of the same material at different temperatures changes due to the phenomenon of thermal and volumetric expansion.
For most substances, including metals, density decreases with increasing temperature.
| All metals have certain physical and mechanical properties, which, in fact, determine their specific gravity. To determine how suitable a particular alloy of ferrous or stainless steel is for production, the specific gravity of rolled metal is calculated. All metal products that have the same volume, but are made from different metals, for example, iron, brass or aluminum, have different mass, which is directly dependent on its volume. In other words, the ratio of the volume of the alloy to its mass—specific density (kg/m3)—is a constant value that will be characteristic of a given substance. The density of the alloy is calculated using a special formula and is directly related to the calculation of the specific gravity of the metal. The specific gravity of a metal is the ratio of the weight of a homogeneous body of this substance to the volume of the metal, i.e. this is density, in reference books it is measured in kg/m3 or g/cm3. From here you can calculate the formula for finding out the weight of a metal. To find this you need to multiply the reference density value by the volume. The table shows the densities of non-ferrous metals and ferrous iron. The table is divided into groups of metals and alloys, where under each name the grade according to GOST and the corresponding density in g/cm3 are indicated, depending on the melting point. To determine the physical value of specific density in kg/m3, you need to multiply the tabulated value in g/cm3 by 1000. For example, this way you can find out what the density of iron is - 7850 kg/m3.
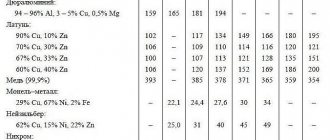
Ferrous metals in the table include iron, manganese, titanium, nickel, chromium, vanadium, tungsten, molybdenum, and ferrous alloys based on them, for example, stainless steel (density 7.7-8.0 g/cm3), black steel ( density 7.85 g/cm3) is mainly used by manufacturers of metal structures in Ukraine, cast iron (density 7.0-7.3 g/cm3). The remaining metals are considered non-ferrous, as well as alloys based on them. Non-ferrous metals in the table include the following types: − light – magnesium, aluminum; − noble metals (precious) - platinum, gold, silver and semi-precious copper; − low-melting metals – zinc, tin, lead. Specific gravity of non-ferrous metals | Atomic weight | Melting point, °C | Specific gravity, g/cc |
| Zinc Zn (Zinc) | 65,37 | 419,5 | 7,13 |
| Aluminum Al | 26,9815 | 659 | 2,69808 |
| Lead Pb (Lead) | 207,19 | 327,4 | 11,337 |
| Tin Sn (Tin) | 118,69 | 231,9 | 7,29 |
| Copper Cu (Copper) | 63,54 | 1083 | 8,96 |
| Titanium Ti (Titanium) | 47,90 | 1668 | 4,505 |
| Nickel Ni (Nickel) | 58,71 | 1455 | 8,91 |
| Magnesium Mg (Magnesium) | 24 | 650 | 1,74 |
| Vanadium V | 6 | 1900 | 6,11 |
| Tungsten W (Wolframium) | 184 | 3422 | 19,3 |
| Chrome Cr (Chromium) | 51,996 | 1765 | 7,19 |
| Molybdenum Mo (Molybdaenum) | 92 | 2622 | 10,22 |
| Silver Ag (Argentum) | 107,9 | 1000 | 10,5 |
| Tantalum Ta (Tantal) | 180 | 3269 | 16,65 |
| Iron Fe (Iron) | 55,85 | 1535 | 7,85 |
| Gold Au (Aurum) | 197 | 1095 | 19,32 |
| Platinum Pt (Platina) | 194,8 | 1760 | 21,45 |
When rolling non-ferrous metal blanks, it is also necessary to know exactly their chemical composition, since their physical properties depend on it.
For example, if aluminum contains impurities (even within 1%) of silicon or iron, then the plastic characteristics of such a metal will be much worse. Another requirement for hot rolled non-ferrous metals is extremely accurate temperature control of the metal.
For example, zinc requires a temperature of strictly 180 degrees when rolling - if it is slightly higher or slightly lower, the capricious metal will sharply lose its ductility.
Copper is more “loyal” to temperature (it can be rolled at 850 - 900 degrees), but it requires that the melting furnace must have an oxidizing (with a high oxygen content) atmosphere - otherwise it becomes brittle.
Specific gravity of aluminum kg m3 – Telegraph
All metals have certain physical and mechanical properties, which, in fact, determine their specific gravity. To determine how suitable a particular alloy of ferrous or stainless steel is for production, the specific gravity of rolled metal is calculated.
All metal products that have the same volume, but are made from different metals, for example, iron, brass or aluminum, have different mass, which is directly dependent on its volume. In other words, the ratio of the volume of the alloy to its mass—specific density (kg/m3)—is a constant value that will be characteristic of a given substance.
The density of the alloy is calculated using a special formula and is directly related to the calculation of the specific gravity of the metal.
The specific gravity of a metal is the ratio of the weight of a homogeneous body of this substance to the volume of the metal, i.e. this is density, in reference books it is measured in kg/m3 or g/cm3. From here you can calculate the formula for finding out the weight of a metal. To find this you need to multiply the reference density value by the volume.
The table shows the densities of non-ferrous metals and ferrous iron.
The table is divided into groups of metals and alloys, where under each name the grade according to GOST and the corresponding density in g/cm3 are indicated, depending on the melting point.
To determine the physical value of specific density in kg/m3, you need to multiply the tabulated value in g/cm3 by 1000. For example, this way you can find out what the density of iron is - 7850 kg/m3.
The most typical ferrous metal is iron. The density value of 7.85 g/cm3 can be considered the specific gravity of iron-based ferrous metal.
Ferrous metals in the table include iron, manganese, titanium, nickel, chromium, vanadium, tungsten, molybdenum, and ferrous alloys based on them, for example, stainless steel (density 7.7-8.0 g/cm3), black steel ( density 7.85 g/cm3) cast iron (density 7.0-7.3 g/cm3) is mainly used. The remaining metals are considered non-ferrous, as well as alloys based on them. Non-ferrous metals in the table include the following types:
− light – magnesium, aluminum;
− noble metals (precious) – platinum, gold, silver and semi-precious copper;
− low-melting metals – zinc, tin, lead.
| Name of metal, designation | Atomic weight | Melting point, °C | Specific gravity, g/cc |
| Zinc Zn (Zinc) | 65,37 | 419,5 | 7,13 |
| Aluminum Al | 26,9815 | 659 | 2,69808 |
| Lead Pb (Lead) | 207,19 | 327,4 | 11,337 |
| Tin Sn (Tin) | 118,69 | 231,9 | 7,29 |
| Copper Cu (Copper) | 63,54 | 1083 | 8,96 |
| Titanium Ti (Titanium) | 47,90 | 1668 | 4,505 |
| Nickel Ni (Nickel) | 58,71 | 1455 | 8,91 |
| Magnesium Mg (Magnesium) | 24 | 650 | 1,74 |
| Vanadium V | 6 | 1900 | 6,11 |
| Tungsten W (Wolframium) | 184 | 3422 | 19,3 |
| Chrome Cr (Chromium) | 51,996 | 1765 | 7,19 |
| Molybdenum Mo (Molybdaenum) | 92 | 2622 | 10,22 |
| Silver Ag (Argentum) | 107,9 | 1000 | 10,5 |
| Tantalum Ta (Tantal) | 180 | 3269 | 16,65 |
| Iron Fe (Iron) | 55,85 | 1535 | 7,85 |
| Gold Au (Aurum) | 197 | 1095 | 19,32 |
| Platinum Pt (Platina) | 194,8 | 1760 | 21,45 |
Table of specific gravity of metal alloys
The specific gravity of metals is most often determined in laboratory conditions, but in their pure form they are very rarely used in construction. Alloys of non-ferrous metals and alloys of ferrous metals, which according to their specific gravity are divided into light and heavy, are much more often used.
Light alloys are actively used by modern industry due to their high strength and good high-temperature mechanical properties. The main metals of such alloys are titanium, aluminum, magnesium and beryllium. But alloys based on magnesium and aluminum cannot be used in aggressive environments and at high temperatures.
Heavy alloys are based on copper, tin, zinc, and lead. Among the heavy alloys, bronze (an alloy of copper with aluminum, an alloy of copper with tin, manganese or iron) and brass (an alloy of zinc and copper) are used in many industries. Architectural parts and sanitary fittings are produced from these grades of alloys.
The reference table below shows the main quality characteristics and specific gravity of the most common metal alloys. The list provides data on the density of the main metal alloys at an ambient temperature of 20°C.
| List of metal alloys | Density of alloys (kg/m3) |
| Admiralty Brass – Admiralty Brass (30% zinc, and 1% tin) | 8525 |
| Aluminum bronze – Aluminum Bronze (3-10% aluminum) | 7700 – 8700 |
| Babbitt – Antifriction metal | 9130 -10600 |
| Beryllium bronze (beryllium copper) – Beryllium Copper | 8100 – 8250 |
| Delta metal – Delta metal | 8600 |
| Yellow brass – Yellow Brass | 8470 |
| Phosphorous bronzes – Bronze – phosphorous | 8780 – 8920 |
| Regular bronzes – Bronze (8-14% Sn) | 7400 – 8900 |
| Inconel | 8497 |
| Incoloy | 8027 |
| Wrought Iron | 7750 |
| Red brass (low zinc) – Red Brass | 8746 |
| Brass, casting – Brass – casting | 8400 – 8700 |
| Brass, rolled – Brass – rolled and drawn | 8430 – 8730 |
Steel classification
Depending on the proportion of non-metallic impurities determined by the method of smelting a given grade, steel alloys are divided into:
- especially high quality;
- high quality;
- ordinary quality.
Based on their chemical composition, alloys are also divided into alloyed and carbon.
Carbon steels
Used primarily for the production of welded structures and contains from 0.25 to 2.14 percent carbon. Within the group, they are further divided into subgroups, and also according to the percentage of carbon:
- high carbon (0.6-2.14);
- medium carbon (0.3-0.55);
- low carbon (below 0.25).
They also contain silicon and manganese as additives. In addition to useful, purposefully introduced additives, the alloy may also contain harmful impurities that negatively affect its physicochemical properties:
- phosphorus reduces ductility when heated and increases brittleness when cooled;
- sulfur leads to the formation of microcracks.
Other impurities may also be present in the alloy.
Alloy steel
In order for the alloy to acquire the required properties during melting, useful additives or alloying elements are added to it, most often metals such as aluminum, molybdenum, chromium, manganese, nickel, vanadium and others.
The properties of the alloy change quite significantly: the alloy acquires resistance to corrosion, special strength, high malleability, increased or decreased electrical conductivity, etc. An alloy with such additives is called alloy steel.
Based on the percentage of alloying additives, they are divided into three groups:
- highly alloyed – over 11;
- medium alloyed – from 4 to 11;
- low alloy – less than 4.
By area of application, steel alloys are divided into:
- tool - high-strength alloys are used for the manufacture of tools, dies, cutters, drills and cutters;
- structural - used for the production of bodies and components of vehicles, machine tools, building structures;
- special. This group includes alloys with increased resistance to acidic and alkaline environments, radiation, stainless alloys, electrical materials, etc.
Some additives and treatments increase the density of the material, while others reduce it, for example:
| Processing method or additive | Density change |
| carbon | is decreasing |
| chrome, aluminum, manganese | is decreasing |
| cobalt, tungsten, copper | growing |
| drawing | grows within three percent |
, please select a piece of text and press Ctrl+Enter.
To quickly search for a specific material or substance, press Ctrl+F.
This page presents a table of densities of basic materials (metals, rubbers, wood, gases, oils) under normal conditions.
| Material | Density, kg/m3 |
| Steel 10 GOST 1050-88 | 7856 |
| Steel 20 GOST 1050-88 | 7859 |
| Steel 40 GOST 1050-88 | 7850 |
| Steel 60 GOST 1050-88 | 7800 |
| S235-S375 GOST 27772-88 | 7850 |
| St3ps GOST 380-2005 | 7850 |
‘);> //–>
‘);> //–>
| Malleable cast iron KCH 70-2 GOST 1215-79 | 7000 |
| High-strength cast iron HF35 GOST 7293-85 | 7200 |
| Gray cast iron SCh10 GOST 1412-85 | 6800 |
| Gray cast iron SCH20 GOST 1412-85 | 7100 |
| Gray cast iron SCh30 GOST 1412-85 | 7300 |
| Silumin AK12zh GOST 1583-93 | 2700 |
| Alloy AK12 GOST 1583-93 | 2710 |
| Alloy AK5M GOST 1583-93 | 2640 |
| Alloy AK7 GOST 1583-93 | 2700 |
| Alloy AO9-1 GOST 14113-78 | 2700 |
| B83 GOST 1320-74 | 7380 |
| B87 GOST 1320-74 | 7300 |
| BN GOST 1320-74 | 9550 |
| Alloy ML10. ML19 GOST 2856-79 | 1810 |
| Alloy VML5 | 1890 |
| Alloy VML9 | 1850 |
| Tin bronze BrO10C10 | 8800 |
| Tin bronze BrO19 | 8600 |
| Tin bronze BrOS10-10 | 9100 |
| Tin bronze BrОA10-1 | 8750 |
| Bronze BrA10Zh3Mch2 GOST 493-79 | 8200 |
| Bronze BrA9ZH3L GOST 493-79 | 8200 |
| Bronze BrMts5 GOST 18175-78 | 8600 |
| Brass L60 GOST 15527-2004 | 8800 |
| Brass LA GOST 1020-97 | 8500 |
| Copper M0, M1, M2, M3 GOST 859-2001 | 8940 |
| Copper MSr1 GOST 16130-90 | 8900 |
| VT1-0 GOST 19807-91 | 4500 |
| VT14 GOST 19807-91 | 4500 |
| VT20L GOST 19807-91 | 4470 |
| F-4 GOST 10007-80 E | 2100 |
| Fluoroplastic – 1 GOST 13744-87 | 1400 |
| Fluoroplastic – 2 GOST 13744-87 | 1700 |
| Fluoroplastic – 3 GOST 13744-87 | 2710 |
| Fluoroplastic – 4D GOST 14906-77 | 2150 |
| Dakryl-2M TU 2216-265-057 57 593-2000 | 1190 |
| Polymethyl methacrylate LPT TU 6-05-952-74 | 1180 |