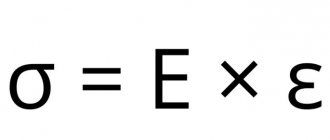Classification
Metallurgists classify metal alloys according to several criteria:
- manufacturing method:
- cast;
- powder;
- production technology:
- foundries;
- deformable;
- powder;
- homogeneity of structure:
- homogeneous;
heterogeneous;
- type of metal - basics:
- black (iron);
- non-ferrous (non-ferrous metals);
- rare metals (radioactive elements);
- number of components:
- double;
- triple;
- and so on;
- physicochemical characteristics:
- refractory;
- fusible;
- high strength;
- heat resistant;
- hard;
- antifriction;
- corrosion-resistant, etc.;
- purpose:
- structural;
- instrumental;
- special.
Types of alloys based on them
Metals and alloys based on them have different physical and chemical characteristics.
The metal having the largest mass fraction is called the base.
Fusibility diagram
Sometimes stops in the temperature drop are also observed on the cooling curve of a solid metal, indicating some processes associated with the release of heat that are already occurring in the solid, for example, a transition from one allotropic form to another.
The cooling curve of an alloy of two metals has a slightly different appearance. Such a curve is shown in Fig. 136 on the right. Point k,
as in the first curve, it corresponds to the beginning of solidification of the alloy, the beginning of the precipitation of crystals of one of the metals included in the alloy.
In this case, the composition of the alloy remaining in the liquid state changes and its solidification temperature continuously decreases during crystallization. However, the heat released during crystallization still slows down the cooling process, as a result of which
a certain break in the curve occurs
k Crystal precipitation and a uniform decrease in temperature occur until the alloy reaches a eutectic composition. Then the temperature drop stops (point k,
since the release of eutectic occurs at a constant temperature. When the release of eutectic ends, the temperature again begins to fall along a smooth curve
cb.
Fig. 136. Plotting a fusibility diagram using cooling curves
Based on a series of curves obtained in this way for various alloys of two metals, a fusibility diagram for a given system is constructed. Its construction for the Bi - Cd system is shown schematically in Fig. 136. Curves 1 and 7
relate to the solidification of pure metals bismuth and cadmium;
all other curves express the cooling of alloys with gradually decreasing bismuth content. Of these, curve 4
corresponds to the solidification of an alloy of eutectic composition (60% Bi and 40% Cd). Fusibility diagrams similar to those considered by us are obtained only in the simplest cases, when the metals being fused do not form either chemical compounds or a solid solution. Examples of such alloys, in addition to those described, are alloys: copper with silver (eutectic contains 28% Cu and 72% Ag), lead with antimony (eutectic with 13% Sb and 87% Pb) and many others.
Fusibility diagrams have a more complex form in cases where two metals, when fused, not only dissolve in each other, but form one or more chemical compounds.
In Fig. 138 shows a diagram of the fusibility of the magnesium-lead system, two substances that form a certain chemical compound Mg2Pb. Here we see two eutectic points - B
and
D,
corresponding to temperatures of 460 and 250°.
The prominent maximum on the ABCDE
(point
C)
corresponds to the melting point of Mg2Pb, and point
M
on the abscissa indicates its composition.
the AB
line , lead along
the ED
, and
Mg2Pb the BCD So, if you cool a liquid alloy containing, say, 40% lead (60% magnesium), then magnesium crystals will first be released from it; as they are released, the temperature will decrease and when it drops to 460°, the entire remaining liquid part of the alloy will begin to solidify at a constant temperature, forming a eutectic mixture of tiny crystals of magnesium and the chemical compound Mg2Pb.
A similar result will be obtained when cooling a liquid alloy containing, for example, 75% lead, but in this case Mg2Pb crystals will be released first. This will happen until the temperature drops to 460° - the point of eutectic formation.
Similar processes with the release of eutectic at 250° occur when the alloy contains more than 80% lead (see CDE
in Fig. 137).
Thus, the left half of the curve from point A
to point C is the fusibility curve of magnesium and Mg2Pb alloys, and the right one - from point
C
to point
E
is the fusibility curve of lead and Mg2Pb alloys.
Properties of alloys
The properties possessed by metal alloys are divided into:
- Structurally insensitive. They are determined by the properties of the components and their percentage. These include :
- density;
- melting temperature;
- thermal and elastic characteristics;
- coefficient of thermal expansion;
- structurally sensitive. Determined by the properties of the element - the base.
- All alloy materials exhibit characteristic metallic properties to one degree or another:
- shine;
- plastic;
- thermal conductivity;
- electrical conductivity.
- In addition, properties are divided into:
- Chemical, determined by the relationship of the material with chemically active substances.
Mechanical, determined by interaction with other physical bodies.
- The main characteristics of alloy materials that influence their suitability for use in a particular engineering structure are:
- Strength is a characteristic of the strength to withstand mechanical loads and destruction.
- Hardness is the ability to resist the penetration of solid bodies into a material.
- Elasticity is the ability to restore the original shape of a body after deformation caused by external load.
- Plasticity is the opposite property of elasticity. Determines the ability of a material to change the shape of a body without its destruction under an applied load and maintaining this new shape.
- Viscosity - the ability to resist rapidly increasing (shock) loads
Mechanical properties
To quantitatively express these properties, special physical quantities and constants are introduced, such as the elastic limit, Hooke's modulus, viscosity coefficient and others.
Main types of alloys
The most numerous types of metal alloys are made based on iron. These are steels, cast irons and ferrites.
Steel is an iron-based substance containing no more than 2.4% carbon, used for the manufacture of parts and housings for industrial installations and household appliances, water, land and air transport, tools and devices. Steels have a wide range of properties. The common ones are strength and elasticity. The individual characteristics of individual steel grades are determined by the composition of alloying additives introduced during smelting. Half of the periodic table is used as additives, both metals and non-metals. The most common of them are chromium, vanadium, nickel, boron, manganese, phosphorus.
Alloy steel
If the carbon content is more than 2.4%, such a substance is called cast iron. Cast iron is more brittle than steel. They are used where it is necessary to withstand large static loads with small dynamic ones. Cast iron is used in the production of frames for large machine tools and technological equipment, bases for work tables, and in the casting of fences, gratings, and decorative items. In the 19th and early 20th centuries, cast iron was widely used in building structures. Cast iron bridges have survived to this day in England.
Cast iron radiators
Substances with a high carbon content and having pronounced magnetic properties are called ferrites. They are used in the production of transformers and inductors.
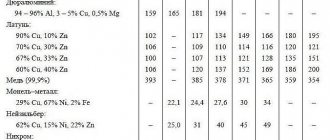
Copper-based metal alloys containing from 5 to 45% zinc are commonly called brasses. Brass is slightly susceptible to corrosion and is widely used as a structural material in mechanical engineering.
Yellow brass
If you add tin to copper instead of zinc, you get bronze. This is perhaps the first alloy deliberately obtained by our ancestors several thousand years ago. Bronze is much stronger than both tin and copper and is second in strength only to well-forged steel.
Lead-based substances are widely used for soldering wires and pipes, as well as in electrochemical products, primarily batteries and accumulators.
Two-component aluminum-based materials, which contain silicon, magnesium or copper, are characterized by low specific gravity and high machinability. They are used in the engine, aerospace, and electrical component and appliance industries.
How are metals produced?
Metals are extracted from ores. Various complex methods and calculation systems are used to determine their contributions. Metal production is carried out in several stages:
- Development of an ore deposit. It can be open or closed. Sometimes extraction methods are combined. The open incision method is less dangerous.
- Ore purification. This process is carried out to extract useful components (ore concentrate) that will be used in further production.
- Metal mining. It is carried out using electrolytic or chemical reduction methods.
- Metal smelting. This is achieved in technological furnaces, where raw materials are heated to elevated temperatures. In addition, a reducing agent is used.
Development of an ore deposit (Photo: Instagram / polyus_official)
Zinc alloys
Zinc-based alloys are characterized by low melting points, corrosion resistance and excellent machinability. They are used in mechanical engineering, the production of computers and household appliances, and in publishing. Good anti-friction properties allow the use of zinc alloys for bearing shells.
Titanium is not the most affordable metal; it is difficult to produce and difficult to process. These shortcomings are compensated by the unique properties of titanium alloys: high strength, low specific gravity, resistance to high temperatures and aggressive environments. These materials are difficult to machine, but their properties can be improved by heat treatment.
Alloying with aluminum and small amounts of other metals increases strength and heat resistance. To improve wear resistance, nitrogen is added to the material or cemented.
Scope of application of titanium alloys
Titanium-based metal alloys are used in the following areas:
- aerospace;
- chemical;
- atomic;
- cryogenic;
- shipbuilding;
- prosthetics.
Metal intensity
Many metals are subject to corrosion, that is, spontaneous destruction as a result of external influences. Enterprises may suffer losses due to corrosion. This is due not only to the high aggressiveness of technological environments and the harsh operating conditions of the equipment, but also to the high metal consumption of the equipment. Metal intensity is the amount of metal that is consumed to create a metal product.
Thus, alloys are used in almost all industries. Homogeneous mixtures of metals have high strength and reliability. They are classified according to various criteria, which makes it possible to increase the efficiency of using alloys. The list of metal alloys is updated every year.
Aluminum alloys
If the first half of the 20th century was the century of steel, then the second was rightly called the century of aluminum.
It is difficult to name a branch of human life in which products or parts made of this light metal would not be found.
Aluminum alloys are divided into:
- Foundry (with silicon). Used to produce conventional castings.
- For injection molding (with manganese).
- Increased strength, with the ability to self-harden (with copper).
Main advantages of aluminum compounds:
- Availability.
- Low specific gravity.
- Durability.
- Cold resistance.
- Good machinability.
- Electrical conductivity.
The main disadvantage of alloy materials is low heat resistance. When reaching 175°C, a sharp deterioration in mechanical properties occurs.
Another area of application is the production of weapons. Aluminum-based substances do not spark under strong friction and collisions. They are used to produce lightweight armor for wheeled and flying military equipment.
Aluminum alloy materials are widely used in electrical engineering and electronics. High conductivity and very low magnetizability make them ideal for the production of housings for various radio and communications devices, computers and smartphones.
Aluminum alloy ingots
The presence of even a small proportion of iron significantly increases the strength of the material, but also reduces its corrosion resistance and ductility. A compromise on iron content is found depending on the requirements for the material. The negative effect of iron is compensated by adding metals such as cobalt, manganese or chromium to the alloy composition.
Magnesium-based materials compete with aluminum alloys, but due to their higher price they are used only in the most critical products.
Table of tensile strength of metals
| Metal | Purpose | Strength, MPa |
| News | Pb | 18 |
| Tin | Sn | 20 |
| Cadmium | Cd | 62 |
| Aluminum | Al | 80 |
| Beryllium | Be | 140 |
| Magnesium | Mg | 170 |
| Copper | Cu | 220 |
| Cobalt | Co | 240 |
| Iron | Fe | 250 |
| Niobium | Nb | 340 |
| Nickel | Ni | 400 |
| Titanium | Ti | 600 |
| Molybdenum | Mo | 700 |
| Zirconium | Zr | 950 |
| Tungsten | W | 1200 |