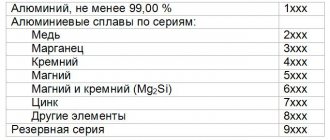
Main characteristics of the mechanical properties of non-ferrous metal alloys
- E - modulus of elasticity - proportionality coefficient between normal stress and elongation;
- G - shear modulus (tangential elastic modulus) - proportionality coefficient between shear stress and relative shear;
- μ - Poisson's ratio - the absolute value of the ratio of transverse to longitudinal deformation in the elastic region;
- σт — yield strength (conditional) — the stress at which the residual deformation after removing the load is 0.2%;
- σв - temporary resistance (tensile strength) - tensile strength;
- δ - relative elongation - the ratio of the absolute residual elongation of the sample after rupture to the initial calculated length;
- hardness (HB, HRC, HV).
What are mechanical properties?
The mechanical properties of aluminum, like other materials, are properties that are associated with the elastic and inelastic response of the material to the application of a load to it, including the relationship between stress and strain. Examples of mechanical properties are:
- modulus of elasticity (tensile, compressive, shear)
- tensile strength (tensile, compressive, shear)
- yield strength
- fatigue limit
- elongation (relative) at break
- hardness.
Mechanical properties are often mistakenly referred to as physical properties.
The mechanical properties of materials, including aluminum and its alloys, that are obtained by tensile testing of the material, such as tensile modulus, tensile strength, tensile yield strength and elongation, are called tensile mechanical properties.
Question 7. Aluminum alloys, and their composition, properties and operating features
For building structures, aluminum alloys containing alloying components and impurities of 5-7% are used (technical aluminum with impurities up to 1% is used very rarely due to its low strength and only for decorative and enclosing elements). Aluminum alloys are divided into deformable
(processed
by pressure
: pressing, drawing, rolling, stamping, etc.), used in building structures, and in
foundries
, used mainly in mechanical engineering.
Aluminum alloys are alloyed
manganese, magnesium, silicon, zinc, copper, chromium, titanium, or several of these components at the same time, depending on which the alloy system receives a name and brand with a symbol.
Aluminum alloys are supplied in various thermal
processing and hardening (hardening, drawing).
Technical aluminum
has very high corrosion resistance, but is low-strength and ductile.
Aluminum-manganese and aluminum-magnesium
the alloys have high corrosion resistance, relatively high strength and are easy to weld.
Multicomponent alloys
have medium and high corrosion resistance, medium and high strength indicators and can be used in welded and riveted load-bearing and enclosing structures.
To improve corrosion resistance, aluminum alloys can be clad
(coated with a thin film of pure aluminum during the manufacture of the semi-finished product).
The structure of aluminum alloys consists of aluminum crystals strengthened by alloying elements (alloying elements enter into solid solution with aluminum and strengthen it).
In Fig. Figure 1 shows diagrams of the tensile performance of some aluminum alloys (for comparison, a curve for steel 3 is also given).
Picture 1
1-technical aluminum AD1M;
2- alloy 1915T; 3- steel 3 The mechanical properties
of aluminum alloys depend not only on the chemical composition, but also on the conditions of their processing. Aluminum alloys have a tensile elasticity modulus E = 0.7∙104 kN/cm2, and a shear elasticity modulus G = 0.27∙104 kN/cm2, which is almost 3 times less than that of steel; therefore, at equal stresses, the deflections of aluminum structures are 3 times greater. Poisson's ratio =0.3. There is no yield plateau on the stress-strain diagram of aluminum alloys. The yield strength is conventionally taken to be the stress at which the relative residual deformation reaches =0.2%. At temperatures above 100 °C there is a slight decrease in strength characteristics, and starting from about 200 °C creep appears. The coefficient of thermal expansion of aluminum = 0.000023, which is 2 times greater than that of steel. At lower temperatures, all mechanical properties of aluminum alloys improve. The impact strength of alloys at normal temperatures is lower than that of steel (about 3.0 kg∙m/cm2), and almost does not decrease at subzero temperatures.
The change in the mechanical properties of aluminum alloys during aging occurs more intensely than that of steel, and the increase in yield strength and strength is much higher. The increase in the strength of aluminum alloys during aging is taken into account when assigning their design resistances. Calculation formulas for aluminum structures under various force influences have the same form as for steel structures. The values of various coefficients are taken depending on the grades of alloys according to the design standards for aluminum structures SNiP II-24-74.
The advantages of aluminum alloys
include: relatively high strength with low density of the material itself; high manufacturability when processed by pressing, rolling or forging, allowing the production of products of complex shapes; high resistance to corrosion, high mechanical characteristics at low temperatures; no sparking during impacts.
Disadvantages of aluminum alloys:
relatively small elastic modulus; high coefficient of thermal expansion; relative complexity of making connections; scarcity and still high cost; low fire resistance.
Aluminum alloy profiles for aluminum structures are produced by rolling, pressing or bending
. Only flat profiles are rolled: sheets, strips, tapes. Pressed profiles can be of very different shapes; their cross-section must fit into a circle with a matrix diameter of 320 mm (there are separate presses with a matrix diameter of 530 mm). These profiles are made on special presses. A cylindrical billet of aluminum alloy heated to approximately 400°C is pressed through a steel matrix with a hole in the shape of the profile section. The matrix is held by a holder. Both solid and hollow (tubular) profiles can be pressed.
Bent profiles are made by bending thin sheets or strips on roller bending machines or press brakes.
QUESTION 8.
Basics of calculation of metal structures.
Design diagram, supporting fastening of elements. Limit states. Groups of limit states. Calculation of structures based on permissible stresses and comparison with calculations based on limit states Basics of calculation of metal structures
The purpose and purpose of structural calculations is
checking the strength, stability and rigidity of the pre-planned structural design of the structure, which makes it possible to clarify the dimensions and ensure the reliability of the structure at the lowest possible cost of metal. Calculation of structures and their structural elements is carried out on the basis of methods of strength of materials and structural mechanics. The main goal of these methods is to determine the internal forces that arise in structures under the influence of applied loads.
The calculation begins with drawing up a design diagram of the structure, temporarily distracting from the actual cross-sectional shape of the elements. The supporting fastenings of the elements are endowed with some theoretical properties (hinged supports, supports with elastic and rigid pinches, etc.). Having determined the forces in the elements according to the accepted design scheme, they select sections, check the load-bearing capacity and design the fastenings in such a way as to satisfy the assigned tasks. Sometimes more accurate methods for determining stresses are necessary, taking into account the development of plastic deformations. However, the mathematical complexity of these methods forces us to often use a number of coefficients in the formulas, the values of which are given in the tables. According to SNiP II-23-81*, building structures are designed to withstand other force impacts based on limit states.
Beyond the limit state
a state of the structure is accepted in which it ceases to satisfy the operational requirements imposed on it, i.e. either loses its ability to resist external influences, or suffers unacceptable deformation or local damage.
studfiles.net
Elastic modulus
The modulus of elasticity, often called Young's modulus, is the ratio of the stress that is applied to a material to the corresponding strain in the range where they are directly proportional to each other.
There are three types of stresses and, accordingly, three types of elastic moduli for any material, including aluminum:
- tensile modulus of elasticity
- compressive modulus of elasticity
- shear modulus of elasticity (shear modulus of elasticity).
Table - Tensile elastic moduli of aluminum and other metals [1]
Yield strength
The stress required to achieve a specified small plastic deformation in aluminum or other material under uniaxial tensile or compressive load.
If the plastic deformation under tensile load is specified as 0.2%, then the term “yield strength 0.2%” (Rp0.2) is used.
Figure 4 – Typical stress-strain diagram for aluminum alloys
ALUMINUM ALLOYS
ALUMINUM ALLOYS, aluminum-based alloys; have low density (up to 3000 kg/m3), high electrical and thermal conductivity, corrosion resistance and specific strength. The first A. s. – alloys of aluminum with silicon, obtained in the 50s. 19th century, had low strength and low corrosion resistance. A turning point in the history of the development of A. with. steel research by A. Wilm (Germany, 1903–11), who discovered in hardened aluminum containing copper and magnesium an increase in strength during the aging process, the so-called. aging effect (see Aging of metals). In 1921, A. Pach (USA) modified the Al–Si alloy by introducing a microscope into it. doses of Na, which led to mean. improving its properties. Later, to obtain A. s. with certain properties they began to use alloying decomp. metals (Cu, Mg, Mn, Si, Zn, Ni, Li, Be, etc.). In Russia in the 1930s–40s. development of A. s. and their introduction into production was carried out by Yu. G. Muzalevsky, S. M. Voronov, I. N. Fridlyander and others.
Until the 1940s Ch. arr. alloys based on the systems Al – Si (silumins), Al – Mg (magnaliums), Al – Cu – Mg (duralumins), Al – Mg – Si (avials). Subsequently, high-strength (based on the systems Al – Zn – Mg, Al – Zn – Mg – Cu, Al – Mg – Si – Cu), heat-resistant (Al – Cu – Mn, Al – Mg – Li, Al – Cu – Mg – Fe – Ni), low density (Al – Be – Mg, Al – Mg – Li, Al – Cu – Li), etc. A.S. Depending on the method of production of semi-finished products and A. products. They are divided into deformable, used for the production of sheets, plates, profiles, pipes, forgings, wire by deformation (rolling, forging, stamping, etc.), and foundry - for the production of shaped products by casting. The composition and some properties of the most common A. s. are given in tables 1, 2 (see page 578).
Wrought alloys in terms of production volume are approx. 80% of all A. s. Chemical and phase composition, thermal regimes. processing of deformable a.s. determined by the need to obtain optimal operating complex and technological properties. Alloys of the Al - Mg system (magnalium) have high corrosion resistance, are well welded, but are not thermally hardened. processing; Sc is added to these alloys to increase strength. Al – Zn – Mg alloys have high strength, are well welded, but at the same time... concentrations of Zn and Mg are prone to delayed corrosion cracking. Al – Mg – Si (avial) alloys combine good corrosion resistance with a pronounced aging effect; amenable to anodizing. Al – Mg – Si – Cu alloys are greatly strengthened as a result of aging, but are characterized by reduced corrosion resistance. Al – Cu – Mg alloys (duralumins) have avg. strength, but high ductility and fracture toughness, low rate of development of fatigue cracks. Al – Zn – Mg – Cu alloys are characterized by the highest strength and yield strength. Al – Mg – Li alloys have the same mechanical properties as duralumin. properties, but lower (by 11%) density and higher elastic modulus. Al – Be – Mg alloys have high specific strength and elasticity modulus, good corrosion resistance, ductility, and good weldability, but due to toxicity their use is limited. Semi-finished products from deformed aluminum s. For subsequent processing, they are obtained from ingots of simple shape - round, flat, hollow.
Table 1. Chemical composition and mechanical properties of some wrought aluminum alloys
| System | Alloy grade | Alloying components (% by weight) | Typical mechanical properties | |||||
| Cu | Mg | Mn | Si | Others | Tensile strength, MPa | Yield strength, MPa | ||
| Al–Mg (magnalia) | AMg6 | < 0,1 | 5,8–6,8 | 0,5–0,8 | ≤ 0,4 | Zn < 0.2; Fe ≤ 0.4 | 340 | 170 |
| 1570 | < 0,1 | 5,3–6,3 | 0,2–0,6 | ≤ 0,2 | Zn < 0.1; Fe ≤ 0.3; Sc 0.25 | 410 | 310 | |
| Al – Mg – Si (aircraft) | AB | 0,1–0,5 | 0,45–0,9 | 0,15–0,35 | 0,5–1,12 | Zn < 0.2; Fe ≤ 0.5; Ti <0.15 | 340 | 280 |
| ADZZ | 0,15–0,4 | 0,8–1,2 | < 0,15 | 0,4–0,8 | Zn < 0.25; Fe ≤ 0.7 | 320 | 260 | |
| Al – Mg – Si – Cu | AK6 | 1,8–2,6 | 0,4–0,8 | 0,4–0,8 | 0,7–1,2 | Zn < 0.3; Fe ≤ 0.7 | 390 | 300 |
| AK8 | 3,9–4,8 | 0,4–0,8 | 0,4–1,0 | 0,6–1,2 | Zn < 0.3; Fe ≤ 0.7 | 470 | 380 | |
| AI – Cu – Mg (duralumins) | D1h | 3,8–4,8 | 0,4–0,8 | 0,4–0,8 | <0,5 | Fe < 0.4 | 380 | 220 |
| D16h | 3,8–4,9 | 1,2–1,8 | 0,3–0,9 | <0,2 | Fe<0.3 | 440 | 300 | |
| Al – Zn – Mg – Cu | V96TS | 2,0–2,6 | 2,3–3,0 | – | <0,3 | Zn 3.0–8.0; Fe < 0.4; Zr 0.1–0.2 | 650 | 620 |
| 1933 | 0,8–1,2 | 1,6–2,2 | – | <0,1 | Zn 6.35–7.2; Fe 0.06–0.15; Zr 0.1–0.18 | 510 | 460 | |
| Al – Cu – Mg – Fe – Ni | AK4–1 | 1,9–2,7 | 1,2–1,8 | ≤ 0,2 | «0,3 | Zn ≤ 0.3; Fe 0.8–1.4; Ni 0.8-1.4 | 420 | 350 |
| Al – Cu – Mn | 1201 | 5,8–6,8 | < 0,02 | 0,2–0,4 | <0,2 | Zn <0.1; Fe ≤ 0.3 | 420 | 320 |
| Al – Mg – Li | 1420 | < 0,05 | 4,5-6,0 | – | <0,15 | Fe ≤ 0.2; Li 1.8–2.3; Zr 0.08–0.15; Na < 0.03 | 430 | 290 |
| 1424 | – | 4,7–5,2 | 0,05–0,25 | ≤ 0,1 | Zn 0.4–0.8; Fe ≤ 0.1; Li 1.5–1.8 | 460 | 320 | |
| Al – Be – Mg | ABM-1 | – | 4,2–5,5 | 0,3 | 0,1 | Fe 0.2; Be 28-32; Ni 0.1 | 430–500 | 250-300 |
| ABM-3 | – | 1,5–2,5 | 0,2 | Fe 0.2; Be 67–72 | 550–620 | 380-480 | ||
| Note. Small additions of Cr, Zr, Sc, Ti, Be, Ca are introduced into a number of alloys. | ||||||||
To deformable A. s. Also include sintered alloys (instead of an ingot, a briquette sintered from powders is used to form products): sintered aluminum powder (SAP) and sintered aluminum alloys (SAS). SAP, strengthened with dispersed particles of aluminum oxide, is superior to all aluminum oxides. by heat resistance. SAS doped with Si, Fe, Ni have a very low coefficient. linear expansion.
Table 2. Chemical composition and mechanical properties of some cast aluminum alloys
| Alloying components (% by weight) | Typical mechanical properties | ||||||||
| System | Alloy grade | Cu | Mg | Mn | Si | Others | Tensile strength, MPa | Yield strength, MPa | |
| Silumins | Al–Si | AK12 (AL2) | 0,6 | – | 0,5 | 13,0 | – | 200 | 110 |
| Al–Si–Mg | AK9ch (AL4) | 0,3 | 0,17–0,3 | 0,2-0,5 | 8,0–10,5 | – | 260 | 200 | |
| AK7ch (AL 9) | 0,2 | 0,2–0,4 | 0,5 | 6,0–8,0 | – | 230 | 130 | ||
| Al–Si–Cu–Mg | AK5M (AL5) | 1,0–1,5 | 0,35–0,6 | 0,5 | 4,5–5,5 | – | 240 | 180 | |
| AK8M3ch (VAL8) | 2,5–3,5 | 0,2–0,45 | – | 7,0–8,5 | Zn 0.5–1.0; Ti 0.1–0.25; B 0.005–0.1; Be 0.05–0.25 | 345 | 290 | ||
| Al–Mg | AMg10 (AL27) | – | 9,5–10,5 | – | – | Zr 0.05–0.20; Ti 0.05–0.15; Be 0.05–0.15 | 314 | 176 | |
| AMg6l (AL23) | 0,15 | 6,0–7,0 | – | – | Zr 0.05–0.20; Ti 0.05–0.15; Be 0.02–0.1 | 225 | 127 | ||
| Al–Cu | AM5 (AL19) | 4,5–5,3 | 0,05 | 0,6–1,0 | 0,3 | Ti 0.15–0.35 | 370 | 260 | |
| AM4.5Kd (VAL10) | 4,5–5,1 | 0,05 | 0,35–0,8 | – | Ti 0.15–0.35; Cd 0.07–0.25 | 420 | 300 | ||
For casting alloys, such characteristics as high fluidity and low tendency to form shrinkage and gas voids, cracks, and cavities are especially important. The highest characteristics are achieved when casting in metal. forms (in a mold, under pressure, during liquid stamping). The most important foundry a.s. – silumins – contain St. 4.5% Si, these include alloys of the Al – Si system and more complex systems: Al – Si – Mg, Al – Si – Cu – Mg; have good casting properties, good corrosion resistance, cf. strength, no shrinkage porosity is formed in castings. Alloys containing Mg St. 5% (alloys of the Al – Mg, Al – Mg – Si systems with the addition of Mn, Be and Ti) are corrosion-resistant, high-strength, highly ductile and have a low density. Prolonged low-temperature (60–80 °C) heating leads to a deterioration in the corrosion resistance of foundry a.s. with high Mg content. The manufacturing technology of these alloys is complex; products are cast. arr. into earthen forms. Alloys with Cu content St. 4% (alloys of the Al – Cu, Al – Cu – Mn systems with the addition of Ti, Cd) are superior in heat resistance to other casting alloys, but have reduced corrosion resistance and casting properties. Casting alloys (except for silumins) are in principle similar to wrought alloys of the corresponding systems, but differ in a higher content of alloying components (Cu, Mg), additives (Ni, Ti) and impurities (Fe).
In addition to the casting methods, the properties of cast alloys are also influenced by the components included in their composition, which are alloying for some alloys, but have a harmful effect on others: Si reduces the strength of Al – Mg alloys; Zn impurity worsens the mechanical properties. properties of Al – Si and Al – Cu alloys; Sn and Pb, even in tenths of a percent, significantly reduce the melting temperature of alloys. Fe has a harmful effect on silumins, causing the formation of brittle inclusions that crystallize in the form of plates. The Fe content depends on the casting method: it is maximum when casting under pressure and in a mold and minimal when casting into the ground. The quality of shaped castings from aluminum s. significantly increases when using a pure charge (reducing the amount of harmful metallic and non-metallic impurities in alloys), modifying alloys (introducing small additives Ti, Zr, Be), using progressive methods of refining and thermal treatment. processing.
A. s. are among the most important structures. materials. In terms of scale of production and consumption, they occupy 2nd place after steel; in industry they use approx. 55 marks A. s. Thanks to the unique exploitation. properties are widely used: in aircraft and rocket production - landing gear, propeller blades, power elements fly. apparatus (skin, fuselage, frames, spars, ribs, upper and lower planes of wings), rocket bodies, fuel and oil tanks; in shipbuilding - ship hulls, deck superstructures, miscellaneous. ship equipment; in the automotive industry - engine parts (pistons, heads, cylinder blocks), cooling radiators, heaters, cabins, bus interiors, tanks for transporting chemicals. and petrochemical products, bulk cargo; in construction - builds. structures, window frames and doors; in the food industry - cans for beer, water, food products, household foil, etc.
Elongation (at break)
Often called "relative elongation". An increase in the distance between two marks on a test specimen that occurs as a result of the specimen deforming under tension until it breaks between the marks.
The amount of elongation depends on the cross-sectional dimensions of the sample. For example, the amount of elongation that is obtained when testing an aluminum sheet specimen will be lower for a thin sheet than for a thick sheet. The same applies to extruded aluminum profiles.
Figure 5 – Influence of alloying elements on strength properties and relative elongation [4]
Extension A
Percentage elongation after specimen rupture at an initial mark spacing of 5.65 √ S0, where S0 is the initial cross-sectional area of the test specimen. The outdated designation of this quantity A5 is currently not used. A similar value in Russian-language documents is designated δ5.
It is easy to check that for round samples this distance between the original marks is calculated as 5·d.
Extension A50mm
The percentage elongation after specimen rupture, relative to the original length between the 50 mm marks and the constant original width of the test specimen (typically 12.5 mm). In the USA, a distance between marks of 2 inches is used, that is, 50.8 mm.
Aluminum as a chemical element
- Aluminum is the third most abundant - after oxygen and silicon - among about 90 chemical elements found in the earth's crust.
- Among the metal elements, it is the first.
- This metal has many useful properties, physical, mechanical, technological - thanks to which it is widely used in all spheres of human activity.
- Aluminum is a malleable metal that has a silvery-white color and is easily processed by most metal forming methods: rolling, drawing, extrusion (pressing), forging.
- Its density - specific gravity - is about 2.70 grams per cubic centimeter.
- Pure aluminum melts at a temperature of 660 degrees Celsius.
- Aluminum has relatively high thermal and electrical conductivity coefficients.
- In the presence of oxygen, it is always covered with a thin, invisible film of oxide. This film is largely impermeable and has fairly high protective properties. Therefore, aluminum generally exhibits stability and long service life under normal atmospheric conditions.
Shear strength
The maximum specific stress, that is, the maximum load divided by the original cross-sectional area that a material can withstand when tested in shear. Shear strength is usually about 60% of tensile strength.
Shear strength is an important quality characteristic of rivets, including aluminum ones.
Figure 6 – Compressive strength, shear strength, load-bearing strength and hardness of various aluminum alloys [4]
Rules for marking aluminum alloys
It is quite difficult to determine the grade of material, so aluminum alloys are marked in such a way that it is clear that this is what they are. A number is assigned to each train. It has an alphanumeric designation.
There are several features inherent in marking:
- At the beginning of the number there are several letters indicating the composition of the material.
- Then comes the digital ordinal code.
- The ending is a number indicating the peculiarities of the treatment (for example, thermal).
To better understand the marking process, consider the example of the D17P alloy. According to the rule, the first letter tells us about the composition of the alloy. D – duralumin. The chemical composition of all duralumin is the same; the differences lie in the concentration of its main elements. The number 17 following the letter D indicates the serial number of a material that has certain qualities. The last letter, in this case P, indicates a semi-hardened alloy. That is, the method of processing the material is pressure without preheating it. Consequently, the strength of the material obtained during such processing will be two times lower than the maximum.
Hardness
The resistance of a metal to plastic deformation, usually measured by making an impression.
Brinell Hardness (HB)
Penetration resistance of a spherical indenter under standardized conditions.
For aluminum and aluminum alloys, the hardness of NV is approximately equal to 0.3 Rm, where Rm is the tensile strength expressed in MPa [2].
If a tungsten carbide indenter is used, the designation HBW is used.
Vickers Hardness (HV)
Penetration resistance of a square pyramid diamond indenter under standardized conditions. Hardness HV is approximately equal to 1.10·HB [2].
Composition and structure of aluminum
To begin with, the structure and chemical composition of aluminum are subject to our consideration. The tensile strength of pure aluminum is extremely small and amounts to up to 90 MPa. If manganese, copper, zinc or magnesium are added to its composition in small proportions, the strength can increase to 700 MPa. The use of special heat treatment will lead to the same result.
The metal with the highest purity (99.99% aluminum) can be used for special and laboratory purposes; in other cases, aluminum with technical purity is used. The most common impurities in it may be silicon and iron, which are practically insoluble in aluminum. As a result of their addition, ductility decreases and the strength of the final metal increases.
The structure of aluminum is represented by unit cells, which in turn consist of four atoms. Theoretically, the density of this metal is 2698 kg/m3.
Now let's talk about the properties of aluminum metal.
This video will tell you about the structure of aluminum:
Fatigue
The tendency of a metal to fail under prolonged cyclic stress that is well below its tensile strength.
Figure 7 – Difference in fatigue behavior of low-carbon steel and aluminum alloys [3]
Fatigue strength
The maximum stress amplitude that a product can withstand for a given number of loading cycles. Typically expressed as the stress amplitude that gives a 50% probability of failure after a given number of loading cycles [2].
Fatigue endurance
The limiting stress below which a material will withstand a specified number of stress cycles [2].