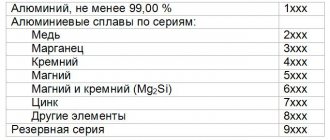
How to age aluminum at home
quote: ! Is this enough to get black?
Sometimes, to install exotic lenses on a camera, you have to order adapters for them from a turner. Mostly they turn me out of duralumin, aluminum and less often from steel. It is known that to increase the contrast in a photograph and to avoid stray light, you need to thoroughly blacken such a homemade adapter.
I have been looking for a long time for an acceptable method of blackening metal that could be used at home and obtain acceptable blackening quality.
The most affordable option seemed to be to buy a can of matte black paint and paint over the necessary parts. But even this method is not so simple. We need to prepare the environment, and definitely not in the apartment, but at least in the garage. And besides, the paint can be easily scratched.
I will generally keep silent about the anodizing method; it requires increased safety precautions and all sorts of experiments with sulfuric acid do not suit me.
Just recently I learned about the method of blackening with ferric chloride. Purely by chance - one person at the market said that he dips shiny parts in waste from etching printed circuit boards and thus gets a good blackening. I thought it was a good idea, but in general it’s not necessary to look for waste, it’s enough just to find ferric chloride (FeCl3) and make the same solution.
I found ferric chloride and ordered it online from a private seller on a bulletin board; a 200 g bag cost me about 50 UAH with postage.
I was pleasantly surprised, since ferric chloride is mainly sold for radio amateurs. I myself used to be interested in radio engineering, about 15 years ago, and I thought that now this industry had long been supplanted by Chinese ready-made radio solutions. It turned out that they were not forced out, since there is a supply for ferric chloride, there is also a demand. But I won’t go off topic, further on…
I ink aluminum, duralumin, steel and brass using this method. And I can say that it worked best with aluminum. The duralumin was slightly worse, but acceptable. The steel did not turn black, but became covered with a coating reminiscent of rust, it stopped shining, at least this way, it was still a little better than it was. The brass changed color a little - it became a little redder, stopped shining, became matte, but did not turn black.
Method of blackening aluminum with ferric chloride
I needed to blacken a couple of duralumin rings for macrofur and a couple of aluminum adapters. For such a small number of parts, 15-20 grams of ferric chloride is sufficient.
Ferric chloride in a container for preparing a solution
First you need to dilute it with a small amount of water. For such a small amount of iron, very little water is needed. It is important that the resulting mixture is thick. so that it does not spread but is spread on the surface. I did it by eye - the thicker the solution, the better.
Ferric chloride solution
While the solution is “infused,” we prepare our parts for blackening. We clean them from possible dirt and dust and degrease them. I just washed them with soap under the tap, that was enough.
Part prepared for blackening
Now that the solution is ready, take some kind of stick. for example, for cleaning ears with cotton wool on the tip. and carefully coat the inner surfaces of the adapter. I only ink them, preferring to leave them shiny on the outside. Make sure that the solution remains on the surfaces and does not run off.
Part with ferric chloride solution applied
Next, it takes a little time for the chemical reaction to take place. The duration of the reaction depends on the proportion of the solution and temperature. If you added warm water, the reaction will go faster.
In my case, the aluminum parts turned black after 7-10 minutes. The duralumin took a little longer to darken, maybe 20 minutes, but I didn’t track the exact time.
The duralumin ring has darkened
As a result, the surface became dark gray and matte. Doesn't glare, which is what we wanted.
If you are not satisfied with the result, you can rinse the parts and go through again with the remaining solution. I did this with duralumin, steel and brass, in the hope that it would turn out better.
Dural began to look noticeably better, steel and brass remained the same. You can also leave them spread for a longer time.
After achieving blackening, the parts can be washed with running water and dried. Then you can use them.
The surface of the same ring after washing and drying. I'm happy with the blackening.
After I blackened the macro bellows ring, which was initially shiny, the contrast in the photos improved a lot, especially when shooting black details with long exposures.
Another aluminum part, blackened using the same method
But what happened to the brass: It didn’t darken at all, but became dull and changed color a little
Here is a relatively simple and high-quality blackening method. I hope that it will be useful not only to me, but also to other enthusiasts.
Do-it-yourself patination (blackening, aging) of copper, brass and bronze
Patination of copper, as well as other methods of its decorative processing (including at home), can make products made from this metal more attractive and give them a touch of noble antiquity. Items made not only from copper, but also from its alloys such as bronze and brass can be subjected to this treatment.
Using various methods of patination of copper alloys, you can achieve completely different shades
Patination and oxidation
The surface of many metals (and copper is one of them), when interacting with the surrounding air and various chemicals, begins to become covered with a thin layer of oxides and oxides. This process, which also leads to a change in the color of the metal surface, is called oxidation. For the most part, the process of metal oxidation occurs naturally, but people have learned to cause it artificially, in industrial or home conditions, which is done to give the product an aged look.
Oxidation should not be confused with patination, a process whose essence lies in the fact that a thin layer of sulfur or chloride compounds is formed on the surface of the metal when interacting with various chemical elements. Patination, which, like oxidation, is accompanied by a change in the color of copper and bronze, can also be performed artificially using special compounds.
Copper aging occurs naturally over time or immediately when the surface is treated with any preparations.
If under natural conditions the process of oxidation and patination of copper or bronze can take years, then when using special solutions, patination occurs in a very short period of time. The surface of a product placed in such a solution literally changes its color before our eyes, acquiring a touch of noble antiquity. Using various chemical compositions, you can perform such procedures as blackening of copper, patination of objects made of copper and bronze, and blackening of brass in production and even at home.
Preparation for processing
Having decided to perform patination or oxidation, you should not only carefully study the question of how to age brass, bronze or blacken copper, but also provide the necessary safety measures.
The vast majority of chemical compounds that are used to carry out such procedures are very toxic and emit vapors that pose a significant danger to human health.
Therefore, to store such substances both in industrial and at home conditions, you should use vessels with well-ground stoppers, which will prevent toxic vapors from entering the surrounding air.
Keep chemicals out of the reach of children
The procedure itself, carried out to change the color of the surface of a product under the influence of chemicals on it, should be performed in a special cabinet to which exhaust ventilation is connected. It should be borne in mind that the doors of such a cabinet should be slightly open during the process of oxidation or patination, which will ensure effective extraction of harmful vapors from its interior.
Products made of copper, brass and bronze should be thoroughly cleaned, degreased and washed in warm water before patination. After the patination or oxidation procedure itself, the treated objects are also washed and placed in sawdust to dry.
Using sawdust is a more gentle drying method, since performing such a procedure with a fabric material can damage the thin film of the formed patina, which has not yet been fixed with varnish.
In addition, using fabric after patination, it is almost impossible to efficiently remove moisture from the recesses on relief surfaces, and sawdust can easily be pulled out.
The varnished surface can be polished with a felt pad.
Changes in color of copper and its alloys from gray to black
The grey, dark gray or black color of copper and its alloys makes the appearance of the product more attractive and presentable. To obtain these colors, the degree of saturation of which can be adjusted, you need the “liver of sulfur” composition that has been used for decades. It got its name due to the fact that during the cooking process it must sinter, that is, turn into a caked mass.
To make such a composition for patination at home, you must perform the following steps:
- one part of powdered sulfur is mixed with two parts of potash;
- the resulting mixture is placed in a tin can, which then must be put on fire;
- After waiting for the powder to melt and start sintering, it is necessary to maintain this process for 15 minutes.
To prepare sulfur liver you will need soda and sulfur
During the sintering process of the powder, a blue-green flame may flare up on its surface, which does not need to be knocked down, since it will not deteriorate the quality characteristics of the sulfur liver. After sintering is completed and completely cooled, the resulting mass should be crushed to a powder state. This powder, if placed in a glass jar with a tight-fitting lid, can be stored for a long time.
In order to patina various metal alloys using liver sulfur, several basic methods are used. Method No. 1
This method involves the use of an aqueous solution of liver sulfur. It can be used to change the color of products made from the following materials:
- copper;
- sterling silver;
- bronze and brass.
The colors that can be used to paint the surfaces of products using this method also vary:
- copper and silver - purple, blue (very difficult to obtain), gray, brown-gray, black;
- brass and bronze – soft golden.
Patination of aluminum at home
Perhaps, every home will certainly have some little thing or product made of aluminum that has already lost its presentable appearance, but you would really like to return it to its former beauty with the always attractive appearance of antiquity. How then? We suggest you do patination of aluminum at home yourself, without resorting to professional help from specialists. Let's look at this process in more detail so that you have a clear idea of what you have to do. Is it difficult? Most often, “aging” aluminum products is practically no more difficult, and sometimes not even easier, than other metals. And all just because aluminum perfectly accepts all kinds of color tones during electrochemical oxidation, or, in other words, anodizing. As a result, a special film is formed on the surface of such a product, preventing the occurrence of metal corrosion, thereby imparting aesthetic charm to the finished product. If you consider this method too complicated for you, then you can use simpler ones, for example, the most ordinary ink for drawing, black alcohol-based varnish or finely crushed graphite and other specialized coloring agents. After such methods, you just need to wipe the “aged” trinket with kerosene. A simple method of patination Having read all the above methods, you still could not choose how best to “age” your new aluminum product, then quickly read this simple method that allows you to patina aluminum at home, using simple improvised means that cost literally pennies. To do this, you will need to carefully wipe the finished aluminum item with a small piece of cloth soaked in acetone to remove all possible “greasy” stains or stubborn dirt that are on the surface. After everything is cleaned and thoroughly dried, you will definitely need to moisten the product with some solvent, for example, White Spirit or any other that you can find at home or buy at a hardware store. Then burn the workpiece with a blowtorch. But, remember! The more solvent you use directly on the product itself, the darker the patina will subsequently appear as a result of its combustion on the surface under the influence of a blowtorch. If you want to get barely noticeable traces of darkening, then it is best to apply the solvent using a brush with coarse bristles. And in order to obtain a very dark shade, the product must be placed completely directly in the solvent. In this case, in convex places it will be necessary to remove its excess so that small accumulations of the substance remain in the recesses. When working in this way, the product is placed in a horizontal position so that the solvent does not flow where it is not needed. You will need to burn with a blowtorch for several minutes, moving the fire evenly to avoid excessive overheating, which could simply melt your aluminum product. After the product has completely cooled, you will also need to carefully lighten its convex parts. To do this, you will need to wipe them with a cotton pad soaked in oil for lubricating a sewing machine, to which the smallest shavings of abrasive powder have been added. Then wipe dry with a clean, dry cloth to remove all abrasive residue. The type and duration of the effect from this method, which allows you to patina aluminum at home, is no worse than from chemical methods. And most importantly, it is much safer - you will not be harmed by acid. Let's summarize Aluminum is a metal with remarkable plastic qualities, which is very convenient to use in creating various decorative parts of the interior. But sometimes you want to replace the novelty of such products with an “antique” that is pleasing to the eye. And we learned how to do this from this article - patination of aluminum at home.
How to “age” metal
At all times, rust has been one of the most serious enemies of iron products. It is very difficult for things damaged by corrosion to regain their attractiveness, and in particularly advanced cases, an item corroded by rust has to be thrown away.
Meanwhile, as strange as it may sound, a red or brown coating on metal can be a welcome phenomenon.
Designers, restorers and other specialists who, by the nature of their activities, come across antiques and their imitation, know this better than anyone else.
The need to “age” objects and their individual parts made of iron and its alloys does not arise very rarely. For example, you have become the owner of a wonderful vintage chest, which is worthy of becoming a real decoration for your living room or office, but has a serious drawback - the absence of one of the lid hinges.
Today, finding or ordering an identical element from a locksmith is not at all difficult, but even a skillfully made replica will not look next to the original, which has a noble antique appearance with a slight touch of corrosion.
Is it possible to process metal so that it looks tens, or even hundreds of years older? Of course you can and we will tell you how to do it without much effort and expense.
Materials and tools
To “aging” metal objects, you do not need either expensive equipment or complex, multi-component chemical compositions. Almost everything you need for work can be found in a toolbox, garage or, as a last resort, in the nearest hardware store:
It is better to do the work outdoors on a sunny day, and this is due not only to the use of reagents that give a violent reaction with the release of gases, but also to the fact that under the influence of direct sunlight the work will progress much faster.
Let's get started
As we have already said, for antique metal processing, it is better to choose a fine sunny day and perform all operations in the open air. But if this is not possible, then make sure that the room where you work is well ventilated or at least ventilated. The process of artificial “rusting” of iron consists of several stages:
- Thoroughly clean the iron object from dust, oils and other contaminants. If its surface is painted, the paint coating should be removed mechanically or chemically.
- Sand the metal surface with sandpaper until it is slightly rough. This will make the metal oxidation reaction more active.
- Place a plastic container on a flat base and place the prepared part in it.
- Protect your eyes with goggles and your hands with rubber gloves. By neglecting protective measures, you expose your body to serious danger.
- Apply vinegar to the item placed in the container using a spray bottle.
- Allow the acid to react with the metal. The appearance of rust will become noticeable within a few minutes.
- Mix two cups of hydrogen peroxide, four tablespoons of vinegar and one and a half teaspoons of table salt in a plastic bottle. Attach the sprayer to the container and begin applying the resulting mixture to the metal. If the proportions are maintained correctly, then the reaction will be instantaneous - bubbles will appear on the surface of the metal, and the rust that has already begun to appear after the initial treatment will become even more active.
- Leave the product to dry with the composition applied to it for 5-10 minutes. If a larger area is being processed, it may take a little longer.
- In order to get a light touch of antiquity, a single treatment is enough. But if you need to get a very rusty thing, the process will have to be repeated 2-4 times.
- After achieving the expected result, remove the item from the plastic container, and dilute the remaining solution in it with water and pour it down the drain.
- To preserve the resulting rust effect, apply a clear acrylic sealant to the “aged” surface. The protective layer will not only prevent rust from staining objects in contact with the part, but also stop the corrosion process, which can continue without your participation and, in the end, completely destroy the item.
The rust obtained as a result of this treatment is indistinguishable from that formed naturally. An acrylic barrier will allow the metal to maintain a stable appearance for many years and at the same time will be invisible.
Useful tips 11/30/2020 11:08:35
Features of heat treatment of aluminum alloys
Aluminum and its alloys require a special approach to heat treatment to achieve a certain strength and structure of the material. Several heat treatment methods are often used. Typically, aging follows after hardening. But some types of materials can be aged without hardening.
This opportunity appears after casting, when the components, at an increased cooling rate, can give the metal the necessary structure and strength. This occurs during casting at a temperature of about 180 degrees. At this temperature, the level of strength and hardness increases, and the degree of ductility decreases.
Each heat treatment method has some features that should be taken into account when processing aluminum products.
Annealing is necessary to impart a uniform structure to the aluminum alloy. Using this method, the composition becomes more homogeneous, the diffusion process is activated and the size of the base particles is equalized. It is also possible to achieve a reduction in the voltage of the crystal lattice. The processing temperature is selected individually, based on the characteristics of the alloy, the required final characteristics and the structure of the material.
Composition and properties of aluminum alloys strengthened by heat treatment
An important step in annealing is cooling, which can be done in several ways. Cooling is usually carried out in an oven or in the open air. Step-by-step combined cooling is also used, first in an oven and then in air.
The characteristics of the finished material directly depend on the rate of temperature decrease. Rapid cooling promotes the formation of supersaturation of the solid solution, and slow cooling promotes a significant level of decomposition of the solid solution.
Quenching is required to strengthen the material by supersaturating the solid solution. This method is based on heating products to temperatures and rapid cooling. This contributes to the complete dissolution of the constituent elements in aluminum. Used for processing wrought aluminum alloys.
To use this method, you need to correctly calculate the processing temperature. The higher the degree, the less time is required for hardening. In this case, it is worth choosing the temperature so that it exceeds the value required for the solubility of the components, but is less than the limit of the metal melt.
The aging method increases the strength of the aluminum alloy. Moreover, it is not necessary to subject the products to artificial aging, since the process of natural aging is possible.
Depending on the type of aging, the rate of structural changes changes. Therefore, artificial aging is more preferable, as it improves productivity. The selection of processing temperature and time depends on the properties of the material and the characteristics of the alloying components.
The right combination of heating level and holding time can increase strength and ductility. This process is called stabilization.
T-states of aluminum alloys
Different options for aging parameters correspond to different designations of the state of aluminum alloys:
- T1 – cooled after pressing to room temperature and naturally aged;
- T4 – after pressing, hardened with separate heating and naturally aged;
- T5 – cooled after pressing to room temperature and artificially aged to maximum strength properties;
- T6 - after pressing, hardened with separate heating and artificially aged to maximum strength properties.
To refer to other aging treatments that are specifically designed to produce mechanical properties that differ from the maximum strength properties. For example, states T52 and T591 are used for aluminum profiles that are subject to bending, and state T7 is used for profiles that are used at elevated temperatures.
Aluminum anodizing methods
Several methods have been developed for processing aluminum alloys, but the chemical method in an electrolyte environment has found widespread use. Acids are used to obtain a solution:
To impart additional properties, salts or organic acids are added to the solution. At home, sulfuric acid is mainly used, but when processing parts with complex configurations, it is preferable to use chromic acid.
The process occurs at temperatures from 0°C to 50°C. At low temperatures, a hard coating forms on the surface of aluminum. As the temperature rises, the process proceeds much faster, but the coating is highly soft and porous.
In addition to the chemical method, in some cases the following methods of aluminum anodization are used:
- microarc;
- color:
- adsorption;
- immersion in electrolyte;
- dipping into a dye solution;
- electroplating;
- interference;
- integral.
Aluminum and aluminum alloys
Aluminum as a structural material is rarely used in its pure form.
Small amounts (sometimes less than 1%) of other elements can significantly change its properties, physical and mechanical. One of the main properties of structural metals is their strength. Unalloyed aluminum has a tensile strength of about 90 MPa. Due to strain hardening (hardening), this value can increase to 200 MPa. However, the addition of small amounts of zinc, copper and magnesium to pure aluminum makes it a high-strength aluminum alloy with a tensile strength of more than 550 MPa. Aluminum alloys are divided into two categories: wrought and cast. Wrought alloys are processed into a given shape by deformation (extrusion, rolling, forging, stamping, drawing). Casting alloys are poured into casting molds.
Annealing methods for aluminum sheets
Annealing of aluminum alloys is not mandatory. But in some cases, without this heat treatment method it is impossible to achieve the desired characteristics of the material.
The reason for the use of annealing may be the special state of the alloy, which can be expressed in a decrease in the ductility of the material.
The use of annealing is recommended when observing three types of conditions:
- The non-equilibrium state characteristic of cast products is associated with the difference in temperature conditions. The cooling rate of cast products significantly exceeds the recommended one, at which the effect of equilibrium crystallization is achieved.
- Plastic deformation. This condition may be caused by technological requirements for the characteristics and shape of the finished product.
- Heterogeneous structure of the material caused by other heat treatment methods, including hardening and aging. In this case, one of the alloying components separates into the intermetallic phase, accompanied by supersaturation of the components.
The above problems can be eliminated by annealing. Normalization of the structure and condition of the aluminum alloy is accompanied by an increase in ductility. Depending on the type of nonequilibrium state, various annealing methods are selected.
Today there are three annealing modes:
- Homogenization. Designed for processing cast ingots. During the heat treatment of ingots at high temperatures, a uniform structure is achieved. This simplifies the rental process while reducing production costs. In some cases it can be used to improve the quality of deformed products. The annealing temperature is maintained within 500 degrees, followed by holding. Cooling can be done in several ways.
- Recrystallization. Used to restore deformed parts. This requires pre-treatment with a press. The annealing temperature varies in the range from 350 to 500 degrees. The holding time does not exceed 2 hours. The speed and method of cooling has no special limits.
- Heterogenization. Additional annealing after other heat treatment methods. This method is necessary for softening aluminum alloys. This processing method makes it possible to reduce the degree of strength while simultaneously increasing the level of ductility. Annealing is carried out at approximately 400 degrees Celsius. Exposure is usually 1-2 hours. This type of annealing significantly improves the performance characteristics of the metal and increases the degree of corrosion resistance.
Press effect
The recrystallization temperature of some aluminum alloys with manganese, chromium, nickel, zirconium, titanium and other transition metals, subjected to certain hot and, in some cases, cold pressure treatment modes, exceeds the usually assigned heating temperature for deformation or hardening. Therefore, after quenching and aging of such alloys, they retain a non-recrystallized (polygonized) structure with a high dislocation density, which significantly increases strength compared to a recrystallized structure. This phenomenon is called structural hardening . Structural hardening is essentially a high-temperature thermo-mechanical treatment.
As a result of structural strengthening, the values of σ0.2 and σв increase to 30 - 40%. Structural hardening is most pronounced in pressed semi-finished products (rods, profiles, pipes), therefore this phenomenon in relation to them is called the press effect.
Source: www.metmk.com.ua
What is the essence of the procedure?
Before you start blackening aluminum at home, you should understand what the meaning of this process is. According to experts, its essence is to create conditions in which a film of iron oxide would form on a metal surface. Depending on which method of aluminum bluing was chosen, its thickness can range from 1 to 10 microns. There are three ways to influence metal. Consequently, blackening of aluminum at home can be thermal, acidic or alkaline. In the first case, the product is heated, and in the other two it is processed in an appropriate solution. During bluing, the tarnish colors on the surface will change. The master only needs to decide on the desired color of oxidation and stop the process in time.
The role of magnesium and silicon in alloys of the 6xxx series
Magnesium and silicon are the main alloying elements in all 6xxx series aluminum alloys. Magnesium and silicon are included in the compound magnesium silicide (Mg2Si) in a ratio of 1.73 to 1 (Figure 1). It is magnesium silicide that makes 6xxx aluminum alloys thermally hardenable. The level of strength properties of these aluminum alloys depends mainly on the number, size and uniformity of distribution of Mg2Si clusters or particles in aluminum.
Based on the magnesium content in the alloy, the amount of silicon that it will “bind” in magnesium silicide is determined: %Si = %Mg/1.73. For example, if the magnesium content in the alloy is 0.45%, then 0.45/1.73 = 0.26% silicon is required to form magnesium silicide. Part of the silicon is associated with iron and manganese in the primary Al(FeMn)Si particles, which are formed during the casting of the pillars. This amount of silicon is estimated as a third or a quarter of the total content of iron and manganese: 1/4 (Fe + Mn). The rest of the silicon is excess.
Hardening of aluminum castings
Hardening is not suitable for all types of aluminum alloys. For a successful structural change, the alloy must contain components such as copper, magnesium, zinc, silicon or lithium. It is these substances that are able to completely dissolve in the composition of aluminum, creating a structure that has properties different from aluminum.
This type of heat treatment is carried out under intense heating, allowing the constituent elements to dissolve in the alloy, with further intensive cooling to their normal state.
Thermal transformations in alloys 6060, 6063, AD31
When choosing a temperature regime, you should focus on the amount of copper. Also, you need to take into account the properties of cast products.
In industrial conditions, the heating temperature for hardening ranges from 450 to 560 degrees. Holding products at this temperature ensures the melting of the components in the composition. The holding time depends on the type of product; for deformed ones it usually does not exceed more than an hour, and for cast ones - from several hours to two days.
The cooling rate during hardening must be selected so that the composition of the aluminum alloy is not subject to decomposition. In industrial production, cooling is carried out using water. However, this method is not always optimal, since when thick products are cooled, an uneven temperature decrease occurs in the center and along the edges of the product. Therefore, for large and complex products, other cooling methods are used, which are selected individually.
Hardening of aluminum profiles on a press
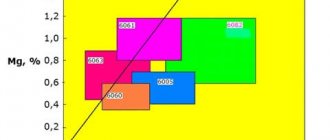
The cooling rate of aluminum profiles - hardening - immediately after exiting the press must be fast enough to retain magnesium and silicon in solid solution.
This ensures the achievement of maximum mechanical properties of the profile material due to their release during subsequent hardening by aging. The required cooling rate of a solid solution of alloying elements - magnesium and silicon in aluminum - to ensure the hardening effect depends on the cross-sectional dimensions of the aluminum profile and the methods of its cooling:
- calm air,
- fans,
- water mist,
- water spray cooling or
- in a stream of water.
The figure and table show the minimum permissible cooling rates of aluminum profiles for various alloys of the 6xxx series. For 6060 alloy aluminum profiles (AD31 aluminum alloy), still air or fan cooling is usually sufficient, while for 6061 alloy profiles, spray water cooling or water flow cooling is required.
Stretching and curing profiles
The usual practice for the manufacture of extruded aluminum profiles includes stretching them from 0.5% to 3% and then curing them with a delay of a day of artificial aging for profiles made of low-alloy 6xxx alloys (no more than 0.9% Mg2Si), for example, aluminum alloys AD31, 6060 and 6063. This helps to achieve optimal mechanical properties of the profiles after aging.
However, such a delay for higher strength aluminum alloys (Mg2Si content more than 0.9%), for example, 6061, can lead to reduced mechanical properties of the aluminum profile material. These alloys contain copper in an amount of at least 0.1%, which counteracts the effect of delayed artificial aging on the final mechanical properties of thermally strengthened aluminum profiles.
Aging of hardened aluminum alloys
After hardening of the aluminum alloy, aging follows, when the alloy is kept at room temperature for several days (natural aging) or for 10–24 hours at elevated temperatures (artificial aging).
During the aging process, the supersaturated solid solution decomposes, which is accompanied by strengthening of the alloy. The decomposition of a supersaturated solid solution, in the lattice of which copper atoms are distributed statistically evenly, occurs in several stages depending on the temperature and duration of aging. During natural (at 20°C) or low-temperature artificial aging (below 100 - 150°C), no decomposition of the solid solution with the release of excess phase is observed; at these temperatures, copper atoms move only within the crystal lattice of the α-solid solution over very short distances and are collected along planes into plate-like formations or disks - Guinier-Preston zones (GP-1). GP-1 zones in Al-Cu alloys with a length of 1–10 nm and a thickness of 0.5–1 nm are more or less evenly distributed within each crystal. The copper concentration in the zones of GP-1 is less than in CuAl2 (54%).
Warm anodizing
The warm anodizing method is used to obtain a base for painting. The coating is porous, but due to this it has high adhesion. Epoxy paint applied on top will reliably protect the aluminum from external influences.
The disadvantage is the low mechanical strength and corrosion resistance of the coating. It is destroyed upon contact with sea water and active metals. This method can be done at home.
The process takes place at room temperature or higher (no more than 50°C). After degreasing, the workpieces are mounted on a suspension that holds them in an electrolyte solution.
Anodizing continues until a milky coating appears on the surface. After removing the stress, the workpieces are washed in cold water. The parts are then painted. They are dyed by placing them in a container of hot dye. After that, the obtained result is consolidated for 1 hour.
AGING IS NOT ALWAYS BAD
The properties of alloys and products made from them depend on many factors and, above all, on the chemical composition. Sometimes adding even a few tenths of a percent of an alloying element makes the alloy extremely hard or, conversely, superplastic. But heat treatment has no less influence. With its help, you can achieve a change not only in the strength or ability of a material to resist fatigue failure, but also in its corrosion resistance. One of the most common methods of processing aircraft materials is called aging.
Science and life // Illustrations
Science and life // Illustrations
Rice. 1. Model of aging of a solid solution of copper in aluminum.
At the beginning of 1924, the first serial domestic all-metal aircraft, ANT-3, designed by A. N. Tupolev, took to the skies.
Rice. 2. Unit cells of stable (θ) and metastable intermediate phases (θ' and θ"), which can be released from the aluminum solution during aging of Al-Cu alloys.
‹
›
In 1909-1911, the German materials scientist A. Wilm, while studying the properties of aluminum, discovered a phenomenon called “natural aging”. It turned out that an aluminum alloy with the addition of 4% copper, 0.5% magnesium and 0.5% manganese after quenching and sharp cooling from a temperature of 500 ° C, being at room temperature for 4-5 days, gradually becomes harder and stronger, without losing plasticity. It would be better to call this process maturation, but the term “aging” has taken root. When aging occurs with heating, it is called artificial aging.
The phenomenon of hardening due to the aging process is of great importance for the development of the aluminum industry.
Research has shown that aging is characteristic not only of Wilm's alloy, but also of many other aluminum alloys. It occurs if the elements introduced into aluminum form an intermetallic compound with each other or with aluminum, that is, a chemical compound of two or more metals that is soluble in aluminum at the hardening temperature and tends to separate from the solid solution as the temperature decreases.
In the aluminum-copper-magnesium system, aluminum forms a compound with copper CuAl2 and a ternary compound with copper and magnesium Al2CuMg, the so-called S phase. Both of these compounds dissolve in aluminum at the quenching temperature; at room temperature their solubility drops sharply, and alloys with these phases are greatly strengthened as a result of the aging process. Industrial production of alloys was first mastered in Germany in the early 20s of the last century. Hence the name “duralumin” or “duralumin”.
It was suggested that during the aging process of hardened duralumin at room temperature, tiny crystals of the CuAl2 compound are released from a supersaturated solid solution of copper in aluminum, strengthening the alloy. Ultimately, the strength of the alloy reaches 36-38 kg/mm2 instead of 7-8 kg/mm2 for pure aluminum.
It seemed that the mechanism of aging had been revealed and the corresponding page in science could be turned. However, in reality, passions were only just flaring up. The fact is that when studying the microstructure through an optical microscope (and the studies were carried out mainly on the aluminum-copper binary alloy, which has lower strength than an alloy containing magnesium and manganese, but is more convenient for study), it was not possible to find CuAl2 particles, and the reality of their existence in a naturally aged alloy began to be questioned. In addition, the release of particles from the solid solution must necessarily reduce the electrical resistance. And in the process of natural aging, both strength and resistance increase in parallel. An increase in electrical resistivity indicated that copper remained within the solid solution.
A heated debate began between supporters and opponents of the hypothesis about the release of copper from aluminum during natural aging.
The study of the aging mechanism was helped by X-ray diffraction analysis and powerful electron microscopes, which allow thin metal films to be viewed through transmission. Everything turned out to be much more complicated than we thought. Copper is not released from the solid solution and does not remain inside it. During the aging process, it collects in disk-shaped areas with a thickness of one to three atomic layers and a diameter of 90 A, forming the so-called Guinier-Preston zones (G.-P. zones). This name of the zones owes its origin to two researchers - J. D. Guinier and A. Preston, who independently discovered copper accumulations in the lattice of an aged aluminum-copper alloy (Fig. 1).
Copper concentration in the G.-P. zones. significantly higher than in the surrounding solid solution, where for each copper atom there are more than 50 aluminum atoms. At a magnification of 500,000 times, the zones appear as blurred lines. Although the entire process of atomic movement takes place within the aluminum lattice, the enrichment of zones with copper causes very specific consequences. Copper has a smaller atomic radius than aluminum, therefore the region of the H.-P. zones. is compressed, and the adjacent areas of the matrix are stretched. Number of zones G.-P. in aluminum-copper alloys there is a huge number: in 1 cm3 their number is equal to the number 5 with 17 zeros (5.1017) (Fig. 1.)
The zones are characterized by the absence of their own lattice and, therefore, a clearly visible boundary between the zone and the solid solution (matrix); they directly transform into each other, there is a coherent connection between them. Natural aging, or more precisely, zone aging, is characterized by average strength and a relatively low yield strength, but high values of fracture toughness and corrosion resistance. This type of aging in industrial alloys is designated by the letter T.
As the aging temperature increases, an intermediate phase θ' appears, which has its own lattice like that of calcium fluoride (Fig. 2) - so-called artificial, or more precisely, phase aging occurs. An important feature of the θ' lattice is the presence in it of planes with a square network of atoms and parameters close to the lattice parameters of the aluminum matrix. Along these planes, the lattice θ' inextricably transforms into the lattice of the aluminum matrix; a coherent connection is maintained here, as is the case with zones. Along other crystalline planes, θ' is separated from the aluminum matrix and interfaces are formed. Phase aging is designated in industrial alloys by the letter T1. It is characterized by maximum strength and yield strength, reduced elongation, fracture toughness and corrosion resistance. With a further increase in the aging temperature or its duration, the phase particles become larger, the coagulation process occurs, the strength and yield strength are somewhat reduced, but corrosion resistance, ductility, and fracture toughness are radically improved. This condition is called coagulative senescence and is designated by the symbols T2 and T3.
With an even greater increase in the heat treatment temperature and slow cooling, a stable phase θ (CuAl2) appears, completely separated from the aluminum matrix in all crystalline regions. Annealing occurs, designated by the letter O or M (soft annealing). The aluminum solid solution passed into a state close to equilibrium, it became plastic, and the strength and electrical resistance decreased. The alloy is easily bent and stamped, but due to its low strength it is not used in structures.
The most common industrial naturally aging alloys such as duralumin grade D16T or 113T (Russia), 2024 (USA) have a strength of 420-450 MPa, a yield strength of 280 MPa, an elongation of 15-20%. Compared to Wilm's alloy, the magnesium content in these alloys is increased from 0.5 to 1.5%. It is from them that the fuselages of passenger aircraft are made in all countries.
The question of the properties of alloys in naturally and artificially aged states arose with particular urgency in 1954 after a series of mysterious crashes of the English passenger plane Comet. In 1949, the English produced the world's first jet four-engine passenger aircraft, the Comet. The cruising speed of this aircraft at an altitude of 12 km was 800 km/h. On January 10, 1954, during a regular flight from Singapore to London near the island of Elba, communication with the plane was suddenly interrupted. By this time, the flight time was 3681 hours.
On April 8, 1954, another Comet plane took off from a Roman airfield, heading for Cairo. After 33 minutes, radio contact with him stopped. The aircraft's flight time was 2,704 hours. The Comet planes never took off again.
The English fleet began searching for the crashed aircraft. The discovered debris was examined and it was determined that the fire started after the planes broke up in the air. When rising to a height, when the external pressure decreased, the fuselage seemed to swell under the influence of constant internal pressure, and when landing on the ground it returned to its original state. This was repeated during each flight cycle. During the total flight time of the Komets, the fuselages were stretched up to 1000 times by internal pressure and compressed during landing. This process led to the formation of a crack. The cracks grew to a critical size, air escaped from the cabin with the force of an explosion, and the entire plane was destroyed. But the hypothesis had to be proven. At the English aviation test center Farnborough, a huge pool was built, into which the entire fuselage of the aircraft fit. After a certain number of cycles of increasing and decreasing pressure, a fatigue crack appeared. It grew and led to the destruction of the aircraft cabin.
This terrible experience of the British served as a lesson for everyone producing aircraft. Huge pools have been built where the pressurized fuselage of each new type of passenger aircraft is tested. Pressure is applied inside it repeatedly, and the same number of times it is reduced, and the number of cycles reaches many thousands and in it has been found that many important industrial aluminum alloys in an artificially aged state become very sensitive to holes, cutouts and other stress concentrators. If a crack occurs in the skin, then in an artificially aged alloy it spreads much faster than after natural aging, therefore the artificially aged alloy is not suitable for the manufacture of pressurized fuselages. All over the world, passenger aircraft fuselages are made only from naturally aged alloys.
At the same time, the pressure in the passenger compartment was slightly reduced - to 0.8 atm., in order to reduce the difference between the external air pressure and the pressure in the passenger compartment. The flight altitude is limited to 10 km, because at an altitude of 12 km turbulent air flows occur especially often.
Soviet duralumin was mastered at the Kolchuginsky plant with the great assistance of the plant’s metallurgist V.A. Butalov, despite the enormous resistance of the majority of domestic aviation specialists. They argued: “Forests in Russia are like a sea, but we will not master duralumin.” However, already at the parade on May 1, 1924, an all-metal Tupolev aircraft was flying in a row of airplanes made of wood, and by 1931, sheets and other products made of duralumin were being produced at full speed by the plant in Setun (now the Kuntsevo district of Moscow) and negotiations were underway with America about purchase of powerful rolling and other equipment for a new modern metallurgical plant in Stupino (not far from Moscow, on the Oka River). In all these matters, A.N. Tupolev and I.I. Sidorin played an important role.
See the issue on the same topic
E. KABLOV - VIAM - a national treasure.
A. ZHIRNOV - Winged metals and alloys.
I. DEMONIS - To the fullest extent.
M. BRONFIN - Testers - researchers and controllers.
Academicians give permission for N. S. Khrushchev’s non-stop flight to New York on an ultra-long-range TU-114 aircraft.
B. SHCHETANOV - Thermal protection of the Buran began with a sheet of tracing paper.
S. MUBOYAJIAN - Plasma versus steam: victory with a clear advantage.
BUREAU OF SCIENTIFIC AND TECHNICAL INFORMATION.
E. KONDRASHOV — Airplanes cannot fly without non-metallic parts.
I. KOVALEV - Into science - from school.
S. KARIMOVA — Corrosion is the main enemy of aviationc.
A. PETROVA - Place it on glue.
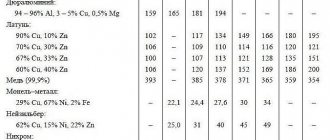
Features of work
Using home bluing is a profitable procedure that allows you to save your family budget. In this case, a special composition is applied to the metal, allowing it to be blackened and forming a protective film.
First, the steel is cleaned and degreased, then the surface is treated using the chosen method.
- All work is done with gloves;
- The bluing solution contains various types of chemicals, so it should be stored in its original packaging and be careful not to spill the substance;
- There should be burn remedies nearby so that first aid can be provided if necessary;
- Work is carried out in a well-ventilated area;
- It is better not to use metal utensils when working.
You will also need sandpaper. It is used for cleaning surfaces. Metal is usually degreased with acetone, perchlorethylene, B-70 gasoline and other organic solvents. Its surface is inert (impervious) to these substances.
Source