A piece of pure aluminum
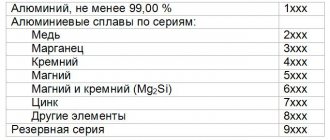
Aluminum
- a very rare mineral of the copper-cupalite family, a subclass of metals and intermetallic compounds of the class of native elements. Mainly in the form of microscopic deposits of a continuous fine-grained structure. It can form lamellar or scaly crystals up to 1 mm, whiskers up to 0.5 mm long are noted. with a thread thickness of several microns. A lightweight paramagnetic metal of silver-white color, easy to form, cast, and machine.
- Structure
- Properties
- Reserves and production
- Origin
- Application
- Classification
- Physical properties
- Optical properties
- Crystallographic properties
See also:
Ice
— structure and physical properties
Copper
— structure and physical properties
STRUCTURE
Cubic face-centered structure. 4 orange atoms
The crystal lattice of aluminum is a face-centered cube, which is stable at temperatures from 4°K to the melting point. There are no allotropic transformations in aluminum, i.e. its structure is permanent. The unit cell consists of four atoms with a size of 4.049596×10-10 m; at 25 °C, the atomic diameter (the shortest distance between atoms in the lattice) is 2.86 × 10-10 m, and the atomic volume is 9.999 × 10-6 m3/g-atom. Impurities in aluminum have little effect on the lattice parameter. Aluminum has great chemical activity; the energy of formation of its compounds with oxygen, sulfur and carbon is very high. In the voltage series, it is among the most electronegative elements, and its normal electrode potential is -1.67 V. Under normal conditions, interacting with atmospheric oxygen, aluminum is covered with a thin (2-10-5 cm) but durable film of aluminum oxide A1203, which protects against further oxidation, which determines its high corrosion resistance. However, if Hg, Na, Mg, Ca, Si, Cu and some other elements are present in aluminum or the environment, the strength of the oxide film and its protective properties are sharply reduced.
PROPERTIES
Native aluminum. Field of view 5 x 4 mm. Azerbaijan, Gobustan region, Caspian Sea, Here-Zira or Bulla Island
Aluminum is a soft, lightweight, silver-white metal with high thermal and electrical conductivity, and is paramagnetic. Melting point 660°C. The advantages of aluminum and its alloys include its low density (2.7 g/cm3), relatively high strength characteristics, good thermal and electrical conductivity, manufacturability, and high corrosion resistance. The combination of these properties allows us to classify aluminum as one of the most important technical materials. It is easily drawn into wire and rolled into thin sheets. Aluminum is chemically active (in air it becomes covered with a protective oxide film - aluminum oxide) and reliably protects the metal from further oxidation. But if aluminum powder or aluminum foil is heated strongly, the metal burns with a blinding flame, turning into aluminum oxide. Aluminum dissolves even in dilute hydrochloric and sulfuric acids, especially when heated. But aluminum does not dissolve in highly diluted and concentrated cold nitric acid. When aqueous solutions of alkalis act on aluminum, the oxide layer dissolves, and aluminates are formed - salts containing aluminum as part of the anion.
Metal density indicator
The density parameter of any substance is calculated as the ratio of mass to volume and measured in g/cm³. Using this indicator for arithmetic calculations allows you to determine the weight of workpieces or products.
Often, to estimate the amount of material per unit volume, the specific gravity indicator is used, which, unlike density, has only a quantitative characteristic.
Aluminum, whose density is 2712 kg/m3, is the most popular material for various industrial sectors. Due to its special physical and chemical characteristics, the metal is used as a alloy component of an alloy with gold.
The melting point is 660 °C. Metal boils at a temperature of 2519 °C. The density of liquid metal is 2560–2640 kg/m3, in the solid state the figure is 2712 kg/m3. Molten chemically pure metal at a temperature of 660 °C has a density of 2.368 g/cm³, and at 1173 °C - 2.304 g/cm³.
Aluminum has high thermal conductivity, which is taken into account along with the physical parameters of the composition. The density of aluminum alloys differs slightly from the density for pure metal.
RESERVES AND PRODUCTION
Aluminum pieces
In terms of prevalence in the Earth's crust, it ranks 1st among metals and 3rd among elements, second only to oxygen and silicon. The mass concentration of aluminum in the earth's crust, according to various researchers, is estimated from 7.45 to 8.14%. The modern production method, the Hall-Héroult process, was developed independently by the American Charles Hall and the Frenchman Paul Héroult in 1886. It consists of dissolving aluminum oxide Al2O3 in molten cryolite Na3AlF6, followed by electrolysis using consumable coke or graphite anode electrodes. This production method requires very large amounts of electricity, and therefore received industrial application only in the 20th century.
Production
aluminum production
One beautiful, but probably implausible legend from “Historia naturalis“
says that one day a jeweler came to the Roman emperor Tiberius (42 BC - 37 AD) with a metal, unbreakable dinner plate, allegedly made from alumina - Al2O3. The plate was very light and shone like silver. By all indications, it should be aluminum. At the same time, the jeweler claimed that only he and the gods knew how to obtain this metal from clay. Tiberius, fearing that metal from easily accessible clay could devalue gold and silver, ordered, just in case, to cut off the man’s head. Obviously, this legend is very doubtful, since native aluminum does not occur in nature due to its high activity, and during the Roman Empire there could not have been technical means that would have made it possible to extract aluminum from alumina.
Only almost 2000 years later - in 1825, the Danish physicist Hans Christian Oersted obtained several milligrams of metallic aluminum, and in 1827 Friedrich Wöhler was able to isolate grains of aluminum, which, however, were immediately covered in air with a thin film of aluminum oxide.
Until the end of the 19th century, aluminum was not produced on an industrial scale.
Only in 1854, Henri Saint-Clair Deville invented the first method of industrial production of aluminum, based on the displacement of aluminum by metallic sodium from double sodium chloride and aluminum NaCl AlCl3. In 1855, the first metal ingot weighing 6-8 kg was obtained. Over 36 years of use, from 1855 to 1890, 200 tons of aluminum metal were produced using the Saint-Clair Deville method. In 1856, he also obtained aluminum by electrolysis of a molten sodium-aluminum chloride.
In 1885, based on the technology proposed by Russian scientist Nikolai Beketov, an aluminum production plant was built in the German city of Gmelingem. Beketov’s technology was not much different from Deville’s method, but it was simpler and involved the interaction between cryolite (Na3AlF6) and magnesium. In five years, this plant produced about 58 tons of aluminum - more than a quarter of all world production of the metal by chemical means in the period from 1854 to 1890.
The method, invented almost simultaneously by Charles Hall in France and Paul Héroux in the USA in 1886 and based on the production of aluminum by electrolysis of alumina dissolved in molten cryolite, laid the foundation for the modern method of aluminum production. Since then, due to improvements in electrical engineering, aluminum production has improved. A notable contribution to the development of alumina production was made by Russian scientists K. I. Bayer, D. A. Penyakov, A. N. Kuznetsov, E. I. Zhukovsky, A. A. Yakovkin and others.
The first aluminum smelter in Russia was built in 1932 in Volkhov. The metallurgical industry of the USSR in 1939 produced 47.7 thousand tons of aluminum, another 2.2 thousand tons were imported.
World War II greatly stimulated aluminum production. Thus, in 1939, its global production, excluding the USSR, was 620 thousand tons, but by 1943 it had grown to 1.9 million tons.
By 1956, the world produced 3.4 million tons of primary aluminum, in 1965 - 5.4 million tons, in 1980 - 16.1 million tons, in 1990 - 18 million tons.
In 2007, the world produced 38 million tons of primary aluminum, and in 2008 - 39.7 million tons. The leaders in production were: China (produced 12.60 million tons in 2007, and 13.50 million tons in 2008), Russia (3.96/4.20), Canada (3.09/3.10), USA (2.55/2.64), Australia (1.96/1.96), Brazil (1.66/ 1.66), India (1.22/1.30), Norway (1.30/1.10), UAE (0.89/0.92), Bahrain (0.87/0.87), South Africa (0.90/0.85), Iceland (0.40/0.79), Germany (0.55/0.59), Venezuela (0.61/0.55), Mozambique (0.56/055 ), Tajikistan (0.42/0.42).
In Russia, the de facto monopolist in aluminum production is Russian Aluminum OJSC, which accounts for about 13% of the world aluminum market and 16% of alumina.
The world's reserves of bauxite are practically limitless, that is, they are incommensurate with the dynamics of demand. Existing facilities can produce up to 44.3 million tons of primary aluminum per year. It should also be taken into account that in the future some of the applications of aluminum may be reoriented to the use of, for example, composite materials.
ORIGIN
Aluminum aggregated with a bayerite crust on the surface. Uzbekistan, Navoi region, Uchkuduk
Due to its high chemical activity, it is not found in pure form, but only as part of various compounds. For example, there are many known ores, minerals, and rocks that contain aluminum. However, it is extracted only from bauxite, the content of which in nature is not very high. The most common substances containing the metal in question are: feldspars; bauxite; granites; silica; aluminosilicates; basalts and others. In small quantities, aluminum is necessarily found in the cells of living organisms. Some species of club mosses and marine inhabitants are capable of accumulating this element inside their bodies throughout their lives.
Additional Information
— Aluminum hydroxide — Encyclopedia of aluminum — Aluminum compounds — International Aluminum Institute
Aluminum, Aluminum, Al (13) Binders containing aluminum have been known since ancient times. However, alum (Latin Alumen or Alumin, German Alaun), which is mentioned, in particular, by Pliny, was understood in ancient times and in the Middle Ages as various substances. In Ruland's Alchemical Dictionary, the word Alumen, with the addition of various definitions, is given in 34 meanings. In particular, it meant antimony, Alumen alafuri - alkaline salt, Alumen Alcori - nitrum or alkali alum, Alumen creptum - tartar (tartar) of good wine, Alumen fascioli - alkali, Alumen odig - ammonia, Alumen scoriole - gypsum, etc. Lemery, the author of the famous “Dictionary of Simple Pharmaceutical Products” (1716), also provides a large list of varieties of alum. Until the 18th century aluminum compounds (alum and oxide) could not be distinguished from other compounds similar in appearance. Lemery describes the alum as follows: “In 1754. Marggraf isolated from an alum solution (by the action of alkali) a precipitate of aluminum oxide, which he called “alum earth” (Alaunerde), and established its difference from other earths. Soon alum earth received the name alumina (Alumina or Alumine). In 1782, Lavoisier expressed the idea that aluminum was an oxide of an unknown element. In his Table of Simple Bodies, Lavoisier placed Alumine among the “simple bodies, salt-forming, earthy.” Here are synonyms for the name alumina: argile, alum. earth, foundation of alum. The word argilla, or argilla, as Lemery points out in his dictionary, comes from the Greek. pottery clay. Dalton in his “New System of Chemical Philosophy” gives a special sign for aluminum and gives a complex structural (!) formula for alum. After the discovery of alkali metals using galvanic electricity, Davy and Berzelius unsuccessfully tried to isolate metallic aluminum from alumina in the same way. Only in 1825 was the problem solved by the Danish physicist Oersted using a chemical method. He passed chlorine through a hot mixture of alumina and coal, and the resulting anhydrous aluminum chloride was heated with potassium amalgam. After evaporation of mercury, writes Oersted, a metal similar in appearance to tin was obtained. Finally, in 1827, Wöhler isolated aluminum metal in a more efficient way - by heating anhydrous aluminum chloride with potassium metal. Around 1807, Davy, who was trying to carry out the electrolysis of alumina, gave the name to the metal supposed to contain it aluminum (Alumium) or aluminum (Aluminum). The latter name has since become common in the USA, while in England and other countries the name Aluminum, later proposed by the same Davy, has been adopted. It is quite clear that all these names come from the Latin word alum (Alumen), about the origin of which there are different opinions, based on the evidence of various authors, dating back to antiquity.
A. M. Vasiliev, noting the unclear origin of this word, cites the opinion of a certain Isidore (obviously Isidore of Seville, a bishop who lived in 560 - 636, an encyclopedist who was engaged, in particular, in etymological research): “Alumen is called a lumen, so how it gives lumen (light, brightness) to paints when added during dyeing." However, this explanation, although very old, does not prove that the word alumen has precisely such origins. Here, only an accidental tautology is quite likely. Lemery (1716) in turn points out that the word alumen is related to the Greek (halmi), meaning salinity, brine, brine, etc. Russian names for aluminum in the first decades of the 19th century. quite varied. Each of the authors of books on chemistry of this period obviously sought to propose its own title. Thus, Zakharov calls aluminum alumina (1810), Giese - alumium (1813), Strakhov - alum (1825), Iovsky - clay, Shcheglov - alumina (1830). In Dvigubsky's Store (1822 - 1830), alumina is called alumina, alumina, alumina (for example, phosphoric acid alumina), and the metal is called aluminum and aluminum (1824). Hess in the first edition of “Foundations of Pure Chemistry” (1831) uses the name alumina (Aluminium), and in the fifth edition (1840) - clay. However, he forms names for salts based on the term alumina, for example, alumina sulfate. Mendeleev in the first edition of “Fundamentals of Chemistry” (1871) uses the names aluminum and clay. In subsequent editions the word gliny no longer appears.
APPLICATION
Aluminum decoration
Widely used as a construction material. The main advantages of aluminum in this quality are lightness, malleability for stamping, and corrosion resistance. The electrical conductivity of aluminum is only 1.7 times less than that of copper, while aluminum is approximately 4 times cheaper per kilogram, but due to its 3.3 times lower density, to obtain equal resistance it needs approximately 2 times less weight . Therefore, it is widely used in electrical engineering for the manufacture of wires, their shielding, and even in microelectronics when depositing conductors on the surface of microcircuit crystals. When aluminum was very expensive, a variety of jewelry was made from it. Thus, Napoleon III ordered aluminum buttons, and in 1889 Mendeleev was presented with scales with bowls made of gold and aluminum. The fashion for jewelry made of aluminum immediately passed when new technologies for its production appeared, reducing the cost many times over. Nowadays, aluminum is sometimes used in the production of costume jewelry.
Aluminum - Al
| Molecular weight | 26.98 g/mol |
| origin of name | from Latin alumen |
| IMA status | approved in 1978 |
Toxicity
It has a slight toxic effect, but many water-soluble inorganic aluminum compounds remain in a dissolved state for a long time and can have a harmful effect on humans and warm-blooded animals through drinking water. The most toxic are chlorides, nitrates, acetates, sulfates, etc. For humans, the following doses of aluminum compounds (mg/kg body weight) have a toxic effect when ingested: aluminum acetate - 0.2-0.4; aluminum hydroxide - 3.7-7.3; aluminum alum - 2.9. Primarily affects the nervous system (accumulates in nervous tissue, leading to severe disorders of the central nervous system). However, the neurotoxicity of aluminum has been studied since the mid-1960s, since the accumulation of the metal in the human body is prevented by its elimination mechanism. Under normal conditions, up to 15 mg of the element per day can be excreted in the urine. Accordingly, the greatest negative effect is observed in people with impaired renal excretory function.
The standard for aluminum content in drinking water is 0.2 mg/l. In this case, this MPC can be increased to 0.5 mg/l by the chief state sanitary doctor for the relevant territory for a specific water supply system.