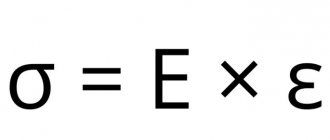Pure copper is used and used for the production of wires, cables, network conductors, and power transmission. The core of the cables is a copper core, in the production of which only very pure metal is used; any impurities reduce the effect of electrical conductivity of copper. Low resistivity allows the use of copper in electrical engineering for the manufacture of power cables and other conductors, as well as power buses carrying high currents; wires made of high-purity copper are used in the windings of electric drives and power transformers.
Copper in everyday life
Currently, when installing electrical wiring in residential premises, only wires with a copper core are allowed, which are made from high-frequency copper wire, which, in turn, is made from class A copper rod. Thus, copper wire is used in our apartments to operate all electrical appliances and lighting. The use of copper instead of aluminum in wires is due not only to the better electrical conductivity of copper, but mainly to better fire safety. Copper is less susceptible to corrosion than aluminum and reacts less with water, which allows the use of copper tubes and pipes to transfer liquids and gases in various home heating and air conditioning systems.
Characteristics of copper
Copper as an electrical conductor
Copper is a metal that belongs to the group of non-ferrous metals, as it has a bright reddish-pink color; with varying degrees of processing, it can have a brown, green, or golden hue. This metal has high electrolytic properties, thermal conductivity, strength and elasticity. Copper is easy to process and is a component of many alloys, thereby increasing its chemical and physical properties. The most famous alloys are bronze - 7 - 10% tin is added to the bulk of copper, copper-nickel alloy - constant (up to 40% nickel in the total mass) and manganin (the alloy includes nickel and manganese). The presence of a large number of distinctive characteristics and the availability of the metal determine the widespread use of copper in various industries, agriculture, construction, and medicine.
Reserves, production
Global quantities of copper ore are estimated at one billion tonnes (explored). The presence of half is confirmed. Scientists believe that the earth's crust hides another three billion tons of copper ore.
Native copper
Countries on all continents have rich reserves:
- America – Chile, Canada, USA.
- Asia – Kazakhstan, Iran.
- Africa – South Africa, Zambia, Zaire.
Russia accounts for 3% of world reserves. The deposits are concentrated in the Urals. The main producer is the Norilsk Nickel concern.
Ore is mined by open or closed methods, depending on the depth of occurrence.
The annual global ore production is 15-20 million tons.
How is copper used in industry?
Copper is smelted in industry
In the production of various products I use copper in its pure form and in the form of alloys with various metals. In its pure form, the metal is used to make network cables and power wires. Copper is distinguished by its ability to conduct electric current quickly and without loss. In this indicator, it is second only to silver, but since it is a precious metal and has a high cost, preference is given to using copper in electrical wiring. To produce the core of cables - the copper core - only pure metal is used; the presence of any impurities significantly reduces the conductive effect. To obtain pure copper, copper blanks are subjected to an electrorefining process. It involves immersing the metal in a bath filled with a solution of copper sulfate, and an electrode connected to electricity is also immersed there. The metal ions move towards the electrode and impurity particles collect near the anode, so they can be removed, resulting in a material containing 99.999% pure copper.
Copper-nickel alloys are characterized by high electrical resistance and are used in instrument making. These alloys are resistant to corrosion and do not break down even in sea water. An alloy in which 40% zinc is called brass, it has increased strength, and its low cost makes it widely used:
After melting copper, they make ingots
- in mechanical engineering;
- in the production of household goods;
- in the chemical industry.
Produced from brass:
- pipes;
- radiators;
- sleeves;
- car accessories and more.
Copper plating is used for chrome plating steel. Steel products are often coated with chromium or nickel for decorative purposes, but this coating is short-lived and may fall off during use. To avoid this, copper plating is applied between the steel and the chrome layer; it provides better adhesion.
The use of copper in industry can also be observed during soldering; it greatly facilitates this process, and the part turns out to be uniform and durable. This metal is quite ductile, it can be used for the manufacture of water pipes of various configurations. In Russia, the use of such pipes is not widespread, but in Europe such products can be found quite often.
Physico-chemical parameters
Copper is a metal with typical external characteristics (brilliance, smoothness) and crystal lattice structure. Endowed with high electrical and thermal conductivity. According to these physical properties, it is second only to silver.
| Name, symbol, number | Copper/Cuprum (Cu), 29 |
| Atomic mass (molar mass) | 63.546(3)a. e.m. (g/mol) |
| Electronic configuration | [Ar] 3d10 4s1 |
| Atomic radius | 128 pm |
| Chemical properties | |
| Covalent radius | 117 pm |
| Ion radius | (+2e) 73 (+1e) 77 (K=6) rm |
| Electronegativity | 1.90 (Pauling scale) |
| Electrode potential | +0.337 V/ +0.521 V |
| Oxidation states | 3, 2, 1, 0 |
| Ionization energy (first electron) | 745.0 (7.72) kJ/mol (eV) |
| Thermodynamic properties of a simple substance | |
| Density (at normal conditions) | 8.92 g/cm³ |
| Melting temperature | 1356.55 K (1083.4 °C) |
| Boiling temperature | 2567 °C |
| Ud. heat of fusion | 13.01 kJ/mol |
| Ud. heat of vaporization | 304.6 kJ/mol |
| Molar heat capacity | 24.44 J/(K mol) |
| Molar volume | 7.1 cm³/mol |
| Crystal lattice of a simple substance | |
| Lattice structure | cubic face-centered |
| Lattice parameters | 3.615 Å |
| Debye temperature | 315 K |
| Other characteristics | |
| Thermal conductivity | (300 K) 401 W/(m K) |
| CAS number | 7440-50-8 |
The main chemical property of metal assessed by humans is zero corrosion. Copper is chemically inactive and does not oxidize under standard conditions.
Copper products in everyday life
This metal is used not only for the production of industrial goods; copper products can also be found in everyday life:
- dishes;
Copper utensils - interior items;
- forged fences;
- sculptures;
- coins.
All these items can be found in almost every home.
Soil fertilizers containing copper sulfate play an important role in agriculture - it stimulates the active growth of various crops, protects them from pests, trees, shrubs, and seeds are treated with a solution of sulfate.
Interior items made of copper
When building houses, copper sheets are used in roofing work. It is known that this metal is resistant to various atmospheric phenomena; under their influence, a protective layer is formed - patina, which has a greenish tint. Patina prevents metal corrosion, and a roof with this coating can last for a long time.
Copper coins
Copper can also be used in electroplating; it has been known since 1873. Electroplating is a special type of art that is based on the electrolytic deposition of metal in an aqueous solution of salts. This method has long gone beyond the boundaries of art and is used in the space industry, aviation, and mechanical engineering. Its essence lies in the fact that the created model of a product, for example, made of plaster or plasticine, is metalized; after removing the model, only the metal form remains. The metallization process occurs by applying a thin layer of metal to the model; graphite is often used, and the workpiece is placed in a solution that contains copper salts. The model plays the role of a cathode and attracts metal particles, which later form the shape of the finished product.
Story
Copper is one of the first metals that humanity dealt with. This was facilitated by the advantages: high prevalence, availability, relatively low melting point.
People appreciated the benefits of copper eight thousand years ago.
The Copper Age began immediately after the Stone Age:
- Copper artifacts dug up in the territory of modern Turkey are recognized as the oldest. These are beads and decorative overlays.
- Cutting tools and utensils were made from metal.
- The history of the discovery of copper mines in Rus' begins in the Urals two thousand years before the new era. Then there were the Caucasus, Altai, Siberia.
- Industrial processing using bronze began in the 14th century. Cannons and bells were cast from the alloy.
The Tsar Bell and the Tsar Cannon were cast from bronze.
It is assumed that the metal is named after the island of Cyprus. Copper deposits were discovered here back in the 3rd century BC, and the population mastered copper smelting.
M. Vasmer’s “Etymological Dictionary of the Russian Language” links the origin of the Russian term copper with the ancient German root smid – blacksmith, metal.
Use of copper in medicine
Traditional medicine considers copper to be a very important element of human life. In the body, this substance is contained in an amount of 2 * 10-4% of the total mass. Every day a person consumes up to 60 mg of copper with food, of which approximately 2 mg is absorbed, which is the necessary norm for a healthy body. Copper plays an important role in the biosynthesis of hemoglobin, in maintaining the levels of sugar, cholesterol and uric acid. Copper is necessary for the normal functioning of the cardiovascular system, brain, and digestive tract. In case of its deficiency, the following develops:
- anemia;
Use of copper in medicine - osteoporosis;
- glaucoma;
- psoriasis;
- bronchial asthma;
- neuritis;
- atrophy of the heart muscle;
- fatigue;
- weight loss;
- accumulation of cholesterol.
Use of copper in medicine:
- For the treatment of acute deficiency, medications containing this microelement are used;
- in therapy - the use of metal applications or bracelets.
The largest amount of microelement is found in foods such as:
- Champignon;
- potato;
- Cod liver;
- whole grain;
- oysters and cuttlefish.
At the same time, excess copper in the body, when its amount exceeds 250 mg, leads to intoxication and disruption of the liver, the development of Wilson's disease, and anemia.
Composition and structure
Copper is a combination of a huge number of crystals of silver, calcium, gold, lead, and nickel. The metals that cuprum consists of are distinguished by ease of processing and relative ductility.
The unit cell of the structural lattice is cubic in shape. Each cell represents a compound of 4 atoms.
During mining, the ore is saturated with a huge amount of impurities. They affect the technical characteristics of the remelted metal and its structure. Common impurities:
- Oxygen is an impurity, the content of which in the composition can reach 0.008%. When exposed to high temperatures, the oxygen content quickly decreases.
- Bismuth is a component that negatively affects the technical characteristics of the finished metal. The permissible amount in the composition is up to 0.001%.
- Manganese has virtually no effect on the properties of cuprum.
- Nickel - reduces thermal conductivity.
- Arsenic - does not affect the properties of the remelted metal. Arsenic neutralizes the negative effects of bismuth, oxygen, and antimony on the final material.
- Tin - enhances thermal conductivity.
- Antimony - reduces thermal and electrical conductivity. Permissible content in the composition is up to 0.05%.
- Sulfur, selenium - reduce the plasticity index if their amount in the composition exceeds 0.001%.
- Zinc has virtually no effect on physical and chemical properties.
- Phosphorus is the main deoxidizing agent. Improves mechanical properties.
The percentage of impurities during production may decrease or increase.
Copper ore
Alloys
Pure copper is not always used in industry. To change the technical characteristics of non-ferrous metal, various components are added to it. The result is alloys that have a copper base. The most common:
- Bronze - Made by adding tin.
- Brass is made from a copper base to which zinc is added.
These are not all compounds where copper is the main component.
Bronze figurine
Areas of application
Areas of application:
- Making tools with thin blades.
- Production of tableware, decorations.
- Manufacturing of wires and radio components.
- Pipe production. Elements for assembling pipelines made from this non-ferrous metal are considered the best when compared with products from other materials.
- Assembly of household appliances.
Copper pipes