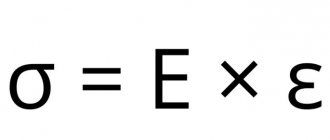In air, copper wires oxidize slowly, becoming covered with a thin layer of CuO oxide, which prevents further oxidation of copper. Copper corrosion is caused by sulfur dioxide SO2, hydrogen sulfide H2S, ammonia NH3, nitric oxide NO, nitric acid vapor and some other reagents.
Conducting copper is obtained from ingots by galvanic purification in electrolytic baths. Impurities, even in insignificant quantities, sharply reduce the electrical conductivity of copper, making it unsuitable for current conductors, therefore only two grades of copper, M0 and M1, are used as electrical copper.
Almost all conductive copper products are made by rolling, pressing and drawing. Thus, wires with a diameter of up to 0.005 mm, tapes with a thickness of up to 0.1 mm, and copper foil with a thickness of up to 0.008 mm can be produced by drawing.
Conducting copper is used both in annealed form after cold working (soft copper grade MM) and without annealing (hard copper grade MT).
At heat treatment temperatures above 900 °C, due to intensive grain growth, the mechanical properties of copper sharply deteriorate.
In order to increase the creep strength and thermal stability, copper is alloyed with silver in the range of 0.07-0.15%, as well as magnesium, cadmium, zirconium and other elements.
Copper with silver additives is used for windings of high-speed and heat-resistant high-power machines, and copper alloyed with various elements is used in commutators and slip rings of heavily loaded machines.
Temperature dependence
Electrical resistivity depends on temperature. But all groups of substances manifest themselves differently when it changes. This must be taken into account when calculating wires that will operate under certain conditions. For example, on the street, where temperature values depend on the time of year, the necessary materials are less susceptible to changes in the range from -30 to +30 degrees Celsius. If you plan to use it in equipment that will operate under the same conditions, then you also need to optimize the wiring for specific parameters. The material is always selected taking into account the use.
In the nominal table, electrical resistivity is taken at a temperature of 0 degrees Celsius. The increase in the indicators of this parameter when the material is heated is due to the fact that the intensity of the movement of atoms in the substance begins to increase. Electric charge carriers scatter randomly in all directions, which leads to the creation of obstacles to the movement of particles. The amount of electrical flow decreases.
As the temperature decreases, the conditions for current flow become better. Upon reaching a certain temperature, which will be different for each metal, superconductivity appears, at which the characteristic in question almost reaches zero.
The differences in parameters sometimes reach very large values. Those materials that have high performance can be used as insulators. They help protect wiring from short circuits and unintentional human contact. Some substances are not applicable at all for electrical engineering if they have a high value of this parameter. Other properties may interfere with this. For example, the electrical conductivity of water will not be of much importance for a given area. Here are the values of some substances with high indicators.
| High resistivity materials | ρ (Ohm m) |
| Bakelite | 1016 |
| Benzene | 1015…1016 |
| Paper | 1015 |
| Distilled water | 104 |
| Sea water | 0.3 |
| Dry wood | 1012 |
| The ground is wet | 102 |
| Quartz glass | 1016 |
| Kerosene | 1011 |
| Marble | 108 |
| Paraffin | 1015 |
| Paraffin oil | 1014 |
| Plexiglass | 1013 |
| Polystyrene | 1016 |
| Polyvinyl chloride | 1013 |
| Polyethylene | 1012 |
| Silicone oil | 1013 |
| Mica | 1014 |
| Glass | 1011 |
| Transformer oil | 1010 |
| Porcelain | 1014 |
| Slate | 1014 |
| Ebonite | 1016 |
| Amber | 1018 |
Substances with low performance are used more actively in electrical engineering. These are often metals that serve as conductors. There are also many differences between them. To find out the electrical resistivity of copper or other materials, it is worth looking at the reference table.
| Low resistivity materials | ρ (Ohm m) |
| Aluminum | 2.7·10-8 |
| Tungsten | 5.5·10-8 |
| Graphite | 8.0·10-6 |
| Iron | 1.0·10-7 |
| Gold | 2.2·10-8 |
| Iridium | 4.74·10-8 |
| Constantan | 5.0·10-7 |
| Cast steel | 1.3·10-7 |
| Magnesium | 4.4·10-8 |
| Manganin | 4.3·10-7 |
| Copper | 1.72·10-8 |
| Molybdenum | 5.4·10-8 |
| Nickel silver | 3.3·10-7 |
| Nickel | 8.7·10-8 |
| Nichrome | 1.12·10-6 |
| Tin | 1.2·10-7 |
| Platinum | 1.07·10-7 |
| Mercury | 9.6·10-7 |
| Lead | 2.08·10-7 |
| Silver | 1.6·10-8 |
| Gray cast iron | 1.0·10-6 |
| Carbon brushes | 4.0·10-5 |
| Zinc | 5.9·10-8 |
| Nikelin | 0,4·10-6 |
Historical reference
According to historical information, the first metals used by man were copper and gold. Both metals are very soft in their pure state, so their use in human life is quite limited. Copper in particular has been used by ancient people since their early use of fire, and since Roman times the metal has been used more intensively in pipes, military weapons, statue decorations, and other uses.
To improve the characteristics of pure metals, for example, greater hardness and strength, over time, man came up with the idea of mixing them. Thus, around 3500 BC, bronze was obtained in Mesopotamia - an alloy of copper and tin, which was highly resistant to corrosion and was stronger than each pure metal separately. Thanks to these properties, bronze began to be used for the production of weapons and tools.
Around 1400 BC, brass was discovered, an alloy of zinc and copper that exhibited excellent resistance to deformation, had high ductility at low and high temperatures, and was highly resistant to corrosion and mechanical wear. However, its use became widespread only in 250 BC with the beginning of coin production in the Roman Empire.
Since that time, the use of brass began to be carried out in a wide variety of areas of human activity, from weapons to jewelry. In the 15th century, it began to be used for the production of astronomical instruments, and with the advent of printing, the alloy began to be actively used in printing. Since the mid-16th century in Europe, bolts and nuts have been made primarily from brass, copper and bronze. This alloy was used to make gears in watch movements, and in the 17th century in Holland, brass was used to make an optical telescope.
What is electrical resistance?
It can be defined based on two positions. The first is related to the formula for Ohm's law. And it sounds like this: electrical resistance is a physical quantity that is defined as the ratio of the voltage in a conductor to the current flowing in it. The mathematical notation is given below.
The second is based on the properties of the body. The electrical resistance of a conductor is a physical quantity that indicates the ability of a body to convert electrical energy into heat. Both of these statements are true. Only in the school course they most often stop at memorizing the first. The quantity being studied is designated by the letter R. The units in which electrical resistance is measured are Ohms.
Relation to conductivity
In isotropic materials, the relationship between resistivity ρ{\displaystyle \rho } and conductivity σ{\displaystyle \sigma } is expressed by the equality
ρ=1σ.{\displaystyle \rho ={\frac {1}{\sigma }}.}
In the case of anisotropic materials, the relationship between the components of the resistivity tensor ρij{\displaystyle \rho _{ij}} and the conductivity tensor σij{\displaystyle \sigma _{ij}} is more complex. Indeed, Ohm's law in differential form for anisotropic materials has the form:
Ji(r→)=∑j=13σij(r→)Ej(r→).{\displaystyle J_{i}({\vec {r}})=\sum _{j=1}^{3}\ sigma _{ij}({\vec {r}})E_{j}({\vec {r}}).}
From this equality and the previously given relationship for Ei(r→){\displaystyle E_{i}({\vec {r}})} it follows that the resistivity tensor is the inverse of the conductivity tensor. Taking this into account, the following holds for the components of the resistivity tensor:
ρ11=1det(σ)σ22σ33−σ23σ32,{\displaystyle \rho _{11}={\frac {1}{\det(\sigma )}},} ρ12=1det(σ)σ33σ12−σ13σ32,{\displaystyle \rho _{12}={\frac {1}{\det(\sigma )}},}
where det(σ){\displaystyle \det(\sigma )} is the determinant of the matrix composed of the components of the tensor σij{\displaystyle \sigma _{ij}}. The remaining components of the resistivity tensor are obtained from the above equations as a result of cyclic rearrangement of the indices 1
,
2
And
3
.
Table of electrical resistivity of some metals
| Wire type | ρ at 20℃, Ohm-m |
| Silver | 1,59×10⁻⁸ |
| Copper | 1,67×10⁻⁸ |
| Gold | 2,35×10⁻⁸ |
| Aluminum | 2,65×10⁻⁸ |
| Tungsten | 5,65×10⁻⁸ |
| Nickel | 6,84×10⁻⁸ |
| Iron | 9,7×10⁻⁸ |
| Platinum | 1,06×10⁻⁷ |
| Steel | 1,6×10⁻⁷ |
| Lead | 2,06×10⁻⁷ |
| Duralumin | 4,0×10⁻⁷ |
| Nichrome | 1,05×10⁻⁶ |
The resistivity is absolutely independent of the shape and size of the conductor, but varies over a wide range when the temperature deviates from the standard value of 20 degrees Celsius. Practical electrical engineering has proven that an increase in temperature increases the resistance of metals to current flow; on the other hand, along with a decrease in temperature, it decreases. It is possible to approximately calculate how significant the change will be, taking into account the fact that all metals have almost the same level of increase in loss of a given value, on average 0.4% per 1°C.
Resistance chart
If this indicator needs to be determined accurately, then you can use this formula:
ρ = ρ0 x (1 + α x (t - t))
, where ρ and ρ0 are, respectively, resistivities at temperatures t and t (20°C, table value), α is the temperature coefficient of resistance.
| Wire type | α |
| Nickel | 0,005866 |
| Iron | 0,005671 |
| Molybdenum | 0,004579 |
| Tungsten | 0,004403 |
| Aluminum | 0,004308 |
| Copper | 0,004041 |
| Silver | 0,003819 |
| Platinum | 0,003729 |
| Gold | 0,003715 |
| Zinc | 0,003847 |
| Steel | 0,003 |
| Nichrome | 0,00017 |
So, for example, having found in the tables the resistivity of copper at 20 degrees Celsius and its temperature coefficient, you can calculate that when heated to 100℃ its resistance will increase by 32%. Almost the same thing will happen with the resistivity of an aluminum cable with the same coefficient (0.004). But the resistivity of steel will increase less significantly - by 24%.
Heat
As the temperature increases, the conductor is saturated with thermal energy, which is transferred to all atoms of the substance. This causes an increase in the intensity of their thermal movement. The latter factor leads to an increase in resistance to the movement of free electrons in a certain direction, since the probability of free electrons meeting atoms increases. When the temperature decreases, fewer atoms can impede the directional movement of electrons, hence the opposite occurs. As a result of a colossal drop in temperature, an interesting phenomenon occurs, called “superconductivity of metals”: resistance decreases to zero in conditions close to absolute zero (-273.15℃). In such conditions, the metal atoms freeze in their positions, and the electrons move without any obstacles.
Superconductivity
How to tell the difference?
Most often you can distinguish by:
- mind;
- weight;
- degree of hardness
without the use of any tools or equipment.
But there are situations when, for accuracy, it is necessary to use :
- reagents,
- tools,
- devices.
Before assessing the scrap that you are going to take to the collection point, you need to clean it of dirt, otherwise you won’t be able to accurately determine it by eye.
By color
Both metals, although to varying degrees, can develop a patina .
Therefore, do not forget to clean the scrap well.
If an object has been in the open air or in water for a long time, the patina layer is difficult to remove.
Sometimes it will be justified to purchase a special cleaning product .
It is advisable to inspect the scrap under a powerful white light.
This implies that one can view either under the sun on a fine day or under a bright fluorescent lamp . Incandescent lamp is not suitable.
Pure copper will have a reddish-brown tint, sometimes with a pink tint. Keep in mind that brass can be red or orange. This type is commonly used for decorations and water pipes.
If the material has an orange, yellow or golden tint, you can be almost sure that it is brass.
If you are engaged in the collection and delivery of scrap metal, then it will be useful for you to know the prices for scrap ferrous metals. If you don’t know where to find ferrous metals, then read this article. Not sure which metal detector model to choose? Check out our review of popular models.
It can also be light golden , pale yellow , and even off-white , but it is very rare for metal detectors, since such an alloy is difficult to process, and it is used mainly in jewelry.
The best recommendation is to carry an item that you are absolutely sure is made of pure copper . You will be able to compare it with the crowbar you found. Most often this method works well.
By sound
Another method that does not require special skills or equipment. You can learn to distinguish metals by sound after a short training. Hit the object with something metal. If it is made of copper, the sound will be muffled and low . This happens because the metal is soft.
Visual inspection and testing for sound and hardness are usually sufficient for field determination.
On the contrary, brass will produce a ringing and high-pitched sound when struck. The second most important inspection method for those who deal with scrap metal is after visual assessment in the light. But this method is justified only with large and voluminous objects - you need something to make a sound.
By hardness
Copper, as mentioned above, is a soft metal. Brass was specifically created to increase the hardness of copper while maintaining some of its other characteristics. Therefore, when damage is caused to scrap, copper will be the material that is more easily deformed . Brass, on the other hand, can withstand impacts .
By labeling
If an item has markings on it, identifying the metal or alloy can be simple and accurate.
As a rule, brass is marked with a mark that begins with the symbol “L” .
Accordingly, copper markings begin with “M” . True, copper quite often does not have any markings.
Here are some transcripts that may be useful:
- Copper markings begin with one letter “M” , followed by numbers. letter “L” on brass products; it can be followed by more letters, and only then by numbers.
- In the United States and Canada, the UNS , according to which brass is marked C2, C3, C4 .
- In the European Union, both metals are marked with the letter C , it all depends on the subsequent letters. For copper they will be B , C, D , and for brass alloy - L, M, N, P and R.
- Not so long ago, labeling consisting of icons of chemical elements was common. For example, Cu Zn (cuprum - zinc) will mean brass.
By weight
Brass is lighter than copper due to the addition of zinc. But in order to determine from a shapeless piece whether it is metal or an alloy, experience is required.
By chips
This test will require a metal drill or access to a machine to get the chips.
With brass it will be, as experts say, needle-shaped , since the material is hard.
It's kind of loose.
With copper, the shavings will be more plastic, so often they don’t even break and the result is ornate , in one continuous spiral.
However, there are grades of brass whose shavings are similar to copper. According to reviews from practitioners, the LS63 grade is a very plastic and tough alloy; after its processing, spiral chips remain.
Acid analysis
If you come across brass grade L-96 , which means the presence of 96% copper in the alloy, it is difficult to distinguish it from the metal without analysis. You can use hydrochloric acid for this. If you drop it on pure copper, it will simply cleanse it of patina and will not react with the metal itself.
If you apply hydrochloric acid to brass, zinc will react and a white oxide will appear on the surface - zinc chloride .
Analyzer
Our portal has detailed material about analyzers of metals and alloys. With the help of such a device you can accurately determine what is in front of you.
Such analyzers have a liquid crystal screen, on which, after interaction between the device and the metal object, a complete list of all constituent elements is displayed.
If it is 99% copper and tenths of a percent of some random impurities, it is copper. If there are other metals in significant quantities - brass. But the method is expensive .
By product type
Some products are made only from copper or only from brass.
This may provide additional guidance.
Tools are made exclusively from brass, which is harder.
Some parts of wind musical instruments are made from copper.
In principle, you need to start from the purpose of the item - if it should be:
- reliable,
- hard,
- stiff,
then, most likely, brass .
If, on the contrary, you need ductility , high electrical or thermal conductivity, then this is copper .
By heating
Another method in which you need to use a gas burner.
The indicator here will be zinc oxide, which forms as a pale white ash-colored coating only on brass if it is heated to temperatures above 600 degrees .
Concept of electrical resistance of a conductor
The classical definition explains electric current by the movement of “free” (valence) electrons. It is provided by the electric field created by the source. Movement in the metal is hampered not only by the normal components of the crystal lattice, but also by defective areas, impurities, and inhomogeneous areas. During collisions with obstacles, due to the transition of momentum into thermal energy, the temperature increases.
A good example is heating water with a boiler.
In gases, electrolytes and other materials the physics of the phenomenon is somewhat different. Linear relationships are observed in metals and other conductors. The basic relationships are expressed by the well-known formula of Ohm's law:
R (electrical resistance) = U (voltage) / I (current).
For convenience, the inverse quantity, conductivity (G = 1/R), is often used. It denotes the ability of a certain material to pass current with certain losses.
To simplify, the example of a water pipe is sometimes used. A moving fluid is an analogue of a current. Pressure is the equivalent of voltage. By decreasing (increasing) the cross section or position of the locking device, the conditions of movement are determined. In a similar way, the basic parameters of electrical circuits are changed using resistance (R).
For your information. The amount of liquid passing per unit time through the control section of the pipe is the equivalent of electrical power.
What and how does resistance depend?
Firstly, from the substance from which the conductor is made. The higher the electrical resistivity value, the worse it will conduct current.
Secondly, on the length of the wire. And here the relationship is direct. As the length increases, the resistance increases.
Thirdly, on thickness. The thicker the conductor, the less resistance it has.
And finally, fourthly, on the temperature of the conductor. And here everything is not so simple. When it comes to metals, their electrical resistance increases as they heat up. The exception is some special alloys - their resistance practically does not change when heated. These include: constantan, nickelin and manganin. When liquids heat up, their resistance decreases.
Areas of use
The use of brass covers a wide variety of areas of human activity. Thus, the golden color of the alloy determined its use in jewelry and various decorative elements. It is also used in boiler making, in the production of military equipment and ammunition, in the manufacture of capacitor wires and tubes, electrical terminals and cash coins.
Due to its resistance to destruction in salt water, the metal is used in the manufacture of equipment for various sea vessels, and its acoustic properties make it possible to make wind instruments: trumpets and accordions. Due to its bactericidal properties, the alloy is used to make door handles in hospitals and clinics.
If we talk about the use as decoration, then we should highlight the production of lamps, lamps, cornices and some jewelry. This kind of thing is produced mainly in the countries of Eastern Europe, in the CIS countries, as well as in many Arab and some Asian countries.
One of the interesting properties of brass, which is unusual for metals, is the absence of sparks when mechanically acting on the product. This unique characteristic makes it possible to use the material as vessels for storing and transporting flammable liquids.
Due to the ease of machining, high wear resistance and low price, the material is used for the manufacture of a variety of valves. Due to its high resistance to corrosion and cavitation, brass is used to make ship propellers. The material is also used in the production of some parts of modern computers.
What is the resistance of copper wire
In metals, a current is formed when an electric field appears. It “forces” electrons to move in an orderly manner, in one direction. Electrons from the distant orbits of an atom, weakly held by the nucleus, form a current.
Copper wires
As negative particles pass through the crystal lattice of copper molecules, they collide with atoms and other electrons. There is an obstacle or resistance to the directional movement of particles.
To evaluate the resistance to current, the value of “electrical resistance” or “electrical impedance” was introduced. It is designated by the letter “R” or “r”. Resistance is calculated using Georg Ohm's formula: R=, where U is the potential difference or voltage acting on a section of the circuit, I is the current strength.
Concept of resistance
Important! The higher the impedance value of a metal, the less current passes through it, and it is copper conductors that are so widespread in electrical engineering due to this property. Based on Ohm's formula, the current value is affected by the applied voltage at constant R
But the resistance of copper wires varies, depending on their physical characteristics and operating conditions.
Based on Ohm's formula, the magnitude of the current is affected by the applied voltage at a constant R. But the resistance of copper wires varies depending on their physical characteristics and operating conditions.
Temperature dependence ρ(T)
For most materials, numerous experiments have been carried out to measure resistivity values. Data for most conductors can be found in reference tables.
Specific resistance of metals and alloys, Ohm*mm2/m
(at T = 20C)
| Silver | 0,016 | Bronze (alloy) | 0,1 |
| Copper | 0,017 | Tin | 0,12 |
| Gold | 0,024 | Steel (alloy) | 0,12 |
| Aluminum | 0,028 | Lead | 0,21 |
| Iridium | 0,047 | Nickelin (alloy) | 0,42 |
| Molybdenum | 0,054 | Manganin (alloy) | 0,45 |
| Tungsten | 0,055 | Constantan (alloy) | 0,48 |
| Zinc | 0,06 | Titanium | 0,58 |
| Brass (alloy) | 0,071 | Mercury | 0,958 |
| Nickel | 0,087 | Nichrome (alloy) | 1,1 |
| Platinum | 0,1 | Bismuth | 1,2 |
Most often, the values of ρ are given at normal, that is, room temperature 20C. But it turned out that with increasing temperature, the resistivity increases linearly in accordance with the formula:
$ ρ(T) = ρ0 * (1 + α*T)$ (6),
where: ρ is the resistivity of the conductor at a temperature of 0C, α is the temperature coefficient of resistivity, which also has its own individual meaning for each substance. From formula (6) it follows that the coefficient α has dimension or .
Rice. 2. Temperature dependence of conductor resistivity
In accordance with the Joule-Lenz law, when an electric current flows, heat is released, which means the temperature of the conductor increases. In addition, depending on the area of application, electrical devices can operate at both low (minus) and high temperatures. For accurate calculations of electrical circuits, it is necessary to take into account the dependence ρ(T). The value of α for a specific material can be found in reference literature.
Rice. 3. Reference values of the temperature coefficient of resistivity of conductors
Brass
| Melting point: 880-950° C | Density: 8300-8700 kg/m³ |
| Specific heat capacity: at 20 °C - 0.377 kJ kg−1 K−1 | Electrical resistivity: (0.07-0.08)×10−6 Ohm m |
General characteristics of brass: brasses are double or multicomponent copper alloys in which zinc is the main alloying component.
Compared to copper, they have higher strength (including at elevated temperatures), corrosion resistance, elasticity, manufacturability (casting, forming, cutting), and tribological characteristics. These are the cheapest and most common copper alloys in mechanical engineering. Double brasses containing up to 20% Zn are called tombak (brasses containing 14-20% Zn are called semi-tompak).
The Cu–Zn phase diagram is characterized by five peritectic reactions. As a result, six different phases crystallize from the liquid solution. Alloys containing up to 50% Zn are of practical importance; The part of the phase diagram corresponding to this content includes the region of the a-solid solution of zinc in copper. The solubility limit of zinc in copper at room temperature is 39%; The a-solid solution has a face-centered crystal lattice. Phase b is a solid solution based on a CuZn compound with a body-centered crystal lattice. The width of the beta-phase homogeneity region varies depending on temperature: from 37 to 57% Zn at high temperatures and from 45 to 49% Zn at room temperature.
According to the phase diagram, double brasses, depending on the structure, are divided into a-brasses, (a + b)-brasses and b-brasses.
At a temperature of 454–468 °C, ordering of the β-solid solution occurs, i.e., below these temperatures, a certain order is observed in the arrangement of copper and zinc atoms in the crystal lattice of the β-phase. The transition of a disordered solid solution to an ordered state is accompanied by a sharp drop in ductility and an increase in the brittleness of the alloys, which makes it difficult to process them under pressure in a cold state.
Thus, brasses containing more than 39% Zn have a two-phase a + b or single-phase structure b and have low ductility, so they are well processed by pressure only in a hot state, in contrast to a-brass, which is well processed in a cold state.
In multicomponent (special) brasses, additives of the third, fourth or more elements can increase strength, hardness, elasticity, corrosion resistance, antifriction properties and technological characteristics. Depending on the additional alloying elements, brass containing A1 is called aluminum; Fe and Mn—ferromanganese; Mn, Sn, Pl - manganese-tin-lead, etc.
Double brasses are marked with the letter L and a number characterizing the average copper content in the alloy in%. In the designation of multicomponent brasses, alloying elements are indicated after the letter L. The numbers after the letters indicate the content of alloying elements.
Based on technological characteristics, brass is divided into cast and pressure-processed. For the production of foundry brass, secondary foundry brass can be used.
Preparation of brass: For smelting brass, any type of smelting furnace used for smelting copper alloys can be used. But it is most advisable to melt brass in electric induction low-frequency furnaces with a magnetic core. Melting brass in electric arc furnaces is less desirable.
When melting copper-zinc alloys, it should be borne in mind that of all other components of the alloy, zinc is the most oxidizable. This is due to its low boiling point.
To reduce zinc oxidation, the following measures are recommended:
1) speed up the process of loading and melting the charge as much as possible, to do this, load the charge into the furnace in a compact form so that the pieces and packages can fit well and tightly into the furnace;
2) the surface of the liquid alloy should be covered with lump charcoal;
3) keep the furnace loading opening always closed if possible;
4) avoid excessive overheating of the melt (above a temperature of 1100-1200 ° C).
Both pure and recycled metals can be used as a charge for smelting brass. When smelting brass using recycled metals, the order in which the charge is loaded into the furnace does not matter much. If there are fresh metals in the charge, copper is first loaded and melted, then recycled metals. Zinc and lead, preheated to 100-120 ° C, are introduced into the melt last. In all cases, smelting is carried out under a layer of charcoal, which is loaded into the furnace with the first portion of the charge.
It is recommended to melt brass from fresh metals and recycled waste in an industrial frequency induction furnace with a magnetic core in the following sequence.
1. Upon completion of casting, the furnace is set to working position. When a bare furnace channel is detected, the current is turned off and the channel is filled with molten metal from another melting furnace.
2. Carefully load two or three packages of waste, turn on the current and further load the charge into the furnace in the following order: first load pre-dried pressed waste in an amount of 15-20% of the mass of the entire charge, shavings, sawdust and other small items; then copper and refractory alloys are loaded into the liquid metal (in the case of melting special brasses); at the same time, the required amount of lump charcoal is loaded into the furnace; after this, the remelted waste and sprues are carefully loaded, and lastly, zinc and other low-melting components are loaded (in the case of preparing special brasses).
3. To avoid damage to the furnace lining, the weight of pieces of charge materials should not exceed 25 kg.
4. The furnace shaft should be loaded tightly and quickly, and the loading window should not remain open for long.
5. When melting, care must be taken to ensure that the charge does not hang in the shaft. A rapid oscillation of the ammeter needle indicates that the charge is separated from the molten metal. The suspended mixture is lowered down using a wooden pole or some other device. When the charge hangs, the smelting time lengthens and the waste of metal increases.
6. In the case of smelting brass on pure metals (copper and zinc), first load 25% of the charge (copper and zinc together), then all the remaining copper and, lastly, zinc (or other low-melting metal).
7. The charge must be dry; Loading wet batch is prohibited.
8. Heavy pieces of charge must be loaded into the furnace using special devices.
9. The charge must be supplied to the furnace in a numbered container (trolley). This eliminates mixing of the charge.
10. It is necessary to have a certain supply of charge near the furnace (two or three carts).
11. After melting and heating the melt to a given temperature, the slag is removed from the surface of the melt, thoroughly mixed and poured.
To increase the fluidity of brass, phosphorus is sometimes added to it before casting in the form of a copper-phosphorus alloy containing 12-14% P.
Melting of silicon and silicon-lead brasses is carried out under a coating flux - glass or borax. Due to the tendency of silicon brasses to absorb reducing gases, they cannot be melted in a reducing atmosphere or under a layer of charcoal.
When melting silicon and silicon-lead brasses, first of all, copper is loaded into a heated furnace, and after it is melted, waste, a copper-silicon alloy, is loaded. Zinc and lead are loaded last after removing the slag from the melt. The melt is thoroughly mixed, brought to the casting temperature and then poured.
Smelting of manganese brass is carried out under conditions of a weakly oxidizing atmosphere or close to neutral under the cover of broken glass flux, or under the cover of charcoal. Manganese is introduced into the melt with alloys after melting all other components of the charge.
Electrical resistivity
Further research made it possible to establish a connection between the value of electrical resistance and its basic geometric dimensions. It turned out that the resistance of the conductor is directly proportional to the length of the conductor L and inversely proportional to the cross-sectional area of the conductor S.
This functional relationship is well described by the following formula:
$ R = ρ *{ L\over S} $ (4)
The constant value ρ for each substance was called resistivity. The value of this parameter depends on the density of the substance, its crystal structure, atomic structure and other internal characteristics of the substance. From formula (4) you can obtain a formula for calculating resistivity if experimental values for R, L and S are available:
$ ρ = R*{ S\over L } $ (5)
For most known substances, measurements were made and entered into reference tables of electrical resistance of conductors.
Specific resistance of metals, Ohm*mm2/m
(at T = 20C)
| Silver | 0,016 | Bronze (alloy) | 0,1 |
| Copper | 0,017 | Tin | 0,12 |
| Gold | 0,024 | Steel (alloy) | 0,12 |
| Aluminum | 0,028 | Lead | 0,21 |
| Iridium | 0,047 | Nickelin (alloy) | 0,42 |
| Molybdenum | 0,054 | Manganin (alloy) | 0,45 |
| Tungsten | 0,055 | Constantan (alloy) | 0,48 |
| Zinc | 0,06 | Titanium | 0,58 |
| Brass (alloy) | 0,071 | Mercury | 0,958 |
| Nickel | 0,087 | Nichrome (alloy) | 1,1 |
| Platinum | 0,1 | Bismuth | 1,2 |
It was experimentally discovered that as the temperature decreases, the resistance of metals decreases. When approaching the temperature of absolute zero, which is -273C, the resistance of some metals tends to zero. This phenomenon is called superconductivity. Atoms and molecules seem to “froze”, stop any movement and offer no resistance to the flow of electrons.
Physical representation
In technical calculations involving the laying of cables of various diameters, parameters are used to calculate the required cable length and its electrical characteristics. One of the main parameters is resistivity. Electrical resistivity formula:
ρ = R * S / l, where:
- ρ is the resistivity of the material;
- R is the ohmic electrical resistance of a particular conductor;
- S—cross section;
- l - length.
The dimension ρ is measured in Ohm•mm2/m, or, to shorten the formula - Ohm•m.
The value of ρ for the same substance is always the same. Therefore, this is a constant characterizing the material of the conductor. It is usually indicated in directories. Based on this, it is already possible to calculate technical quantities.
It is important to say about specific electrical conductivity. This value is the inverse of the resistivity of the material, and is used equally with it. It is also called electrical conductivity. The higher this value, the better the metal conducts current. For example, the conductivity of copper is 58.14 m/(Ohm•mm2). Or, in SI units: 58,140,000 S/m. (Siemens per meter is the SI unit of electrical conductivity).
Metal resistance table
To be convinced of the benefits of copper, it is necessary to make an appropriate comparative analysis. Below are the metal resistance values in the summary table.
Basic electrical parameters of conductors made from different materials
| Material | Resistivity in Ohms per meter measured at room temperature (+20°C) | Specific electrical conductivity under similar conditions, in siemens per meter |
| Copper | 1.68x10^-3 | 5.96x10^7 |
| Silver | 1.59x10^-3 | 6.3x10^7 |
| Gold | 2.44x10^-3 | 4.1x10^7 |
| Aluminum | 2.82x10^-3 | 3.5x10^7 |
| Tungsten | 5.6x10^-3 | 1.79x10^7 |
| Iron | 1x10^-7 | 1x10^7 |
| Platinum | 1.06x10^-7 | 9.43x10^6 |
| Lithium | 9.28x10^-8 | 1.08x10^7 |
Important! The low resistance of an iron conductor is not enough for the widespread use of corresponding products in practice. Active oxidation provokes rapid destruction
Thin films
The resistance of thin flat films (when its thickness is much less than the distance between the contacts) is usually called “resistivity per square”, RSq.{\displaystyle R_{\mathrm {Sq} }.} This parameter is convenient because the resistance of a square piece of conducting film is not depends on the size of this square, when voltage is applied on opposite sides of the square. In this case, the resistance of a piece of film, if it has the shape of a rectangle, does not depend on its linear dimensions, but only on the ratio of the length (measured along the current lines) to its width L/W
: RSq=RWL,{\displaystyle R_{\mathrm {Sq} }=RW/L,} where
R
is the measured resistance. In general, if the shape of the sample is different from rectangular and the field in the film is non-uniform, the van der Pauw method is used.
Similarities and differences
Brass alloy consists mostly of copper, so it is natural that they are similar not only visually, but also in some properties. The more copper in the alloy, the more similar their colors will be. This is where the exact coincidences end.
Visually, less than 80% copper are easily distinguished . They are slightly similar to gold, as they have a pronounced yellow tint. The more zinc, the lighter the shade.
Because of this, brass is even used to counterfeit or imitate gold . Copper has a main shade of reddish, which often has a pink tint.
With a strong decrease in temperature, brass does not lose its relatively limited ductility and does not become brittle . Conducts electricity and heat worse.
They differ in such a way as hardness .
Copper is softer and more ductile , while brass, on the contrary, is hard and it is difficult to give it any shape without annealing.
The shavings are also different: for brass they are needle- shaped , for copper they are twisted into a spiral .
Let's look at the properties that brass and copper have and whether they have any differences:
| Copper | Brass |
| Plastic, soft | Solid |
| Reddish-brown-pink tint | Golden tone |
| Lower sound on impact | Alt |
| Heavy | Easier |
| The shavings are twisted into a spiral | Needle shavings |