In many branches of modern industry, a material such as copper is very widely used. The electrical conductivity of this metal is very high. This explains the expediency of its use primarily in electrical engineering. Copper produces conductors with excellent performance characteristics. Of course, this metal is used not only in electrical engineering, but also in other industries. Its demand is explained, among other things, by its qualities such as resistance to corrosion damage in a number of aggressive environments, refractoriness, ductility, etc.
Historical reference
Copper is a metal known to man since ancient times. The early acquaintance of people with this material is explained primarily by its wide distribution in nature in the form of nuggets. Many scientists believe that copper was the first metal recovered by man from oxygen compounds. Once upon a time, rocks were simply heated over a fire and cooled sharply, causing them to crack. Later, the reduction of copper began to be carried out on fires with the addition of coal and blowing with bellows. The improvement of this method ultimately led to the creation of the shaft furnace. Even later, this metal began to be produced by the method of oxidative smelting of ores.
What kind of wiring is needed for an apartment?
In Soviet times, aluminum cables were used for wiring. The most powerful consumers of electricity were washing machines and refrigerators. They took a couple of hundred watts from the network. Aluminum coped with such low loads with a bang.
Now people use electric kettles (2 kW), vacuum cleaners (1-2 kW) and other powerful household appliances. Aluminum wires in such conditions overheat and burn out. Therefore, in a modern apartment you can only use copper wiring.
Additional Information. Regardless of whether aluminum or copper wiring is used, it is worth considering the insulation material. There must be compliance with fire safety requirements. Insulation is made from non-combustible materials. These standards are especially controlled in crowded places.
Copper: electrical conductivity of the material
In a quiet state, all free electrons of any metal revolve around the nucleus. When an external source of influence is connected, they line up in a certain sequence and become current carriers. The degree to which a metal can pass through itself is called electrical conductivity. Its unit of measurement in the International SI is Siemens, defined as 1 cm = 1 ohm -1.
The electrical conductivity of copper is very high. In this indicator, it surpasses all base metals known today. Only silver passes current better than it. The electrical conductivity of copper is 57x104 cm -1 at a temperature of +20 °C. Due to this property, this metal is currently the most common conductor of all those used for industrial and domestic purposes.
Copper perfectly withstands constant electrical loads and is also reliable and durable. Among other things, this metal is also characterized by a high melting point (1083.4 °C). And this, in turn, allows copper to work in a heated state for a long time. In terms of prevalence as a current conductor, only aluminum can compete with this metal.
Resistivity of various materials
We can talk about resistivity only in the presence of elements that conduct current, since dielectrics have infinite or close to infinite electrical resistance. In contrast, metals are very good conductors of current. You can measure the electrical resistance of a metal conductor using a milliohmmeter, or an even more accurate microohmmeter. The value is measured between their probes applied to the conductor section. They allow you to check circuits, wiring, windings of motors and generators.
Metals vary in their ability to conduct current. The resistivity of various metals is a parameter that characterizes this difference. The data is given at a material temperature of 20 degrees Celsius:
- Silver (ρ = 0.01498 Ohm•mm2/m);
- Aluminum (ρ = 0.027);
Copper (ρ = 0.01721);- Mercury (ρ = 0.94);
- Gold (ρ = 0.023);
- Iron (ρ = 0.1);
- Tungsten (ρ = 0.0551);
- Brass (ρ = 0.026...0.109);
- Bronze (ρ = 0.095);
- Steel (ρ = 0.103…0.14);
- An alloy of nickel, manganese, iron and chromium - nichrome (ρ = 1.051...1.398).
The parameter ρ shows what resistance a meter conductor with a cross section of 1 mm2 will have. The higher this value, the greater the electrical resistance of the desired wire of a certain length. The smallest ρ, as can be seen from the list, is silver; the resistance of one meter of this material will be equal to only 0.015 Ohms, but this is too expensive a metal to use on an industrial scale. Next comes copper, which is much more common in nature (not a precious metal, but a non-ferrous metal). Therefore, copper wiring is very common.
The influence of impurities on the electrical conductivity of copper
Of course, in our time, much more advanced techniques are used to smelt this red metal than in ancient times. However, even today it is almost impossible to obtain completely pure Cu. Copper always contains various types of impurities. This could be, for example, silicon, iron or beryllium. Meanwhile, the more impurities in copper, the lower its electrical conductivity. For the manufacture of wires, for example, only sufficiently pure metal is suitable. According to regulations, copper with an amount of impurities not exceeding 0.1% can be used for this purpose.
Very often this metal contains a certain percentage of sulfur, arsenic and antimony. The first substance significantly reduces the ductility of the material. The electrical conductivity of copper and sulfur is very different. This impurity does not conduct current at all. That is, it is a good insulator. However, sulfur has virtually no effect on the electrical conductivity of copper. The same applies to thermal conductivity. With antimony and arsenic the opposite picture is observed. These elements can significantly reduce the electrical conductivity of copper.
Main characteristics of copper:
- Specific weight equal to 8.93 g/cm3;
- Conductivity: Responsible for the transfer of current from one point to another. The higher the conductivity of a metal, the better it transmits electricity. At +20 degrees, the conductivity of copper is 59.5 million siemens per meter (S/m);
- Electrical resistance, specific at 20°C, equal to 0.0167 Ohm x mm2/m;
- Melting point equal to 1083°C.
Various electrical products: cable cores and wires, electrical busbars and transformer windings are made from various types of copper.
Methods for producing electrical copper
Electrical copper is an extremely pure metal, since any impurity sharply reduces electrical conductivity.
- So, just 0.02% of aluminum impurity, although it is also a conductor, will lead to a decrease in conductivity by 9-10%, but what can we say about impurities that are not conductors at all, so technological defects here are simply unacceptable.
- in the presence of 0.1% phosphorus, the resistance increases by 55%, therefore the conductivity decreases as the reciprocal of the resistance;
- if there is bismuth or lead in copper in an amount of more than 0.001%, then this causes red brittleness (cracking during hot pressure treatment);
- Oxygen in copper makes soldering difficult and increases resistivity. To avoid this, a phosphorus additive is introduced;
- hydrogen - forms microcracks and increases fragility.
If several impurities are present, then there are situations where they interact and their influence increases significantly.
For the use of copper to transmit electricity, the presence of impurities has only a negative effect.
To obtain sufficiently pure electrical copper, a method called electrorefining, based on electrolysis, is used. Conditions are created under which impurities are separated from the copper molecules deposited on one of the electrodes, resulting in the output being electrolytic copper with a purity of 99.999%, necessary for electrical needs.
Another important area is the production of alloys based on or with the addition of copper. It is noteworthy that rather soft copper with many other metals forms not soft, but hard alloys - solutions in which the atoms of different metals are distributed relatively evenly.
Adding a small amount of arsenic to red copper, a product of fire refining, significantly increases its strength, but impairs its weldability.
Copper alloys used in electrical engineering
Brasses - Alloys of copper and zinc, widely used in electrical engineering. Brass is used for spring contacts and plug connectors.
In various brands of brass, the zinc content can reach up to 43%. Brasses containing up to 39% zinc have a single-phase solid solution structure and are called a-brasses. These brasses have the greatest ductility, so they are used to make parts by hot or cold rolling and drawing: sheets, strips, wire. Without heating, parts of complex configurations can be made from sheet brass using deep drawing and stamping.
Brasses with a zinc content of over 39% are called a+b-brasses or two-phase and are used mainly for shaped castings.
Two-phase brass is harder and more brittle and can only be pressed when hot.
The addition of tin, nickel and manganese to brass increases the mechanical properties and anti-corrosion resistance, and the addition of aluminum in composition with iron, nickel and manganese gives the brass, in addition to improving the mechanical properties and corrosion resistance, high hardness. However, the presence of aluminum in brasses makes soldering difficult, and soldering with soft solders becomes almost impossible.
· brass grades L68 and L63
Due to their high ductility, they are easily stamped and can be bent; they are easily soldered with all types of solders. In electrical engineering they are widely used for various live parts;
· brass grades LS59-1 and LMTs58-2
used for the manufacture of rotor (squirrel) cages of electric motors and for current-carrying parts made by cutting and hot stamping; soldered well with various solders;
· brass LA67-2.5
used for cast current-carrying parts of increased mechanical strength and hardness that do not require soldering with soft solders;
· brass LK80-3L and LS59-1L
widely used for cast current-carrying parts of electrical equipment, for brush holders and for casting rotors of asynchronous motors. They accept soldering well with various solders.
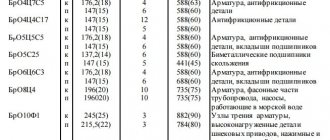
Conductor bronzes are copper alloys, the need for their use is mainly caused by the insufficient mechanical strength and thermal stability of pure copper in some cases. Copper alloys with tin, aluminum, silicon, and lead. The general range of bronzes is very extensive, but only a few brands of bronzes have high electrical conductivity.
· cadmium bronze
refers to the most common conductor bronzes. Of all the grades, cadmium bronze has the highest electrical conductivity. Due to its increased abrasion resistance and higher heat resistance, this bronze is widely used for the manufacture of trolley wires and collector plates;
· beryllium bronze
refers to alloys that gain strength as a result of aging. It has high elastic properties, stable when heated to 250 °C, and electrical conductivity is 2-2.5 times greater than the conductivity of other grades of general-purpose bronzes. This bronze has found wide application for the manufacture of various spring parts that simultaneously act as a current conductor, for example: current-carrying springs, certain types of brush holders, sliding contacts in various devices, plug connectors, etc.;
· Phosphor bronze
has high strength and good spring properties; due to its low electrical conductivity, it is used for the manufacture of spring parts with low current densities.
Cast current-carrying parts are made from various grades of machine-building cast bronzes with a conductivity within the range of 8-15% of the conductivity of pure copper. A characteristic feature of bronzes is low shrinkage compared to cast iron and steel and high casting properties, therefore they are used for casting various current-carrying parts of complex configurations intended for electrical machines and apparatus.
All brands of cast bronzes can be divided into tin and tin-free, where the main alloying elements are Al, Mn, Fe, Pb, Ni.
Manganin is an alloy of copper with the addition of manganese and nickel. Used for the manufacture of additional resistors and shunts in measuring technology.
Alloy characteristics
The electrical conductivity of metals can depend not only on the amount of impurities present in them, but also on other indicators. For example, as the heating temperature increases, the ability of copper to pass current through itself decreases. Even the method of its manufacture affects the electrical conductivity of such wire. In everyday life and in production, both soft annealed copper conductors and hard-drawn ones can be used. The first variety has a higher ability to pass current through itself.
However, the additives used and their quantity have the greatest influence on the electrical conductivity of copper. The table below provides the reader with comprehensive information regarding the current carrying capacity of the most common alloys of this metal.
Electrical conductivity of copper alloys
Condition (O - annealed, T - hard-drawn)
Tin bronze (0.75%)
Cadmium bronze (0.9%)
Aluminum bronze (2.5% A1, 2% Sn)
Phosphor bronze (7% Sn, 0.1% P)
The electrical conductivity of brass and copper is comparable. However, for the first metal this figure is, of course, slightly lower. But at the same time it is higher than that of bronzes. Brass is used quite widely as a conductor. It passes current worse than copper, but at the same time it costs less. Most often, contacts, clamps and various parts for radio equipment are made from brass.
Conducting Bronzes
Conducting bronzes belong to copper alloys, the need for their use is mainly caused by the insufficient mechanical strength and thermal stability of pure copper in some cases.
The general range of bronzes is very extensive, but only a few brands of bronzes have high electrical conductivity.
· cadmium bronze
refers to the most common conductor bronzes. Of all the grades, cadmium bronze has the highest electrical conductivity. Due to its increased abrasion resistance and higher heat resistance, this bronze is widely used for the manufacture of trolley wires and collector plates;
· beryllium bronze
refers to alloys that gain strength as a result of aging. It has high elastic properties, stable when heated to 250 °C, and electrical conductivity is 2-2.5 times greater than the conductivity of other grades of general-purpose bronzes. This bronze has found wide application for the manufacture of various spring parts that simultaneously act as a current conductor, for example: current-carrying springs, certain types of brush holders, sliding contacts in various devices, plug connectors, etc.;
· Phosphor bronze
has high strength and good spring properties; due to its low electrical conductivity, it is used for the manufacture of spring parts with low current densities.
Cast current-carrying parts are made from various grades of machine-building cast bronzes with a conductivity within the range of 8-15% of the conductivity of pure copper. A characteristic feature of bronzes is low shrinkage compared to cast iron and steel and high casting properties, therefore they are used for casting various current-carrying parts of complex configurations intended for electrical machines and apparatus.
All brands of cast bronzes can be divided into tin and tin-free, where the main alloying elements are Al, Mn, Fe, Pb, Ni.
Relationship with thermal conductivity coefficient
The specific electrical conductivity of copper is 59,500,000 S/m. This indicator, as already mentioned, is correct, however, only at a temperature of +20 o C. There is a certain connection between the thermal conductivity coefficient of any metal and specific conductivity. It is established by the Wiedemann-Franz law. It is performed for metals at high temperatures and is expressed in the following formula: K/γ = π 2 / 3 (k/e) 2 T, where y is the specific conductivity, k is the Boltzmann constant, e is the elementary charge.
Of course, a similar connection exists for a metal such as copper. Its thermal conductivity and electrical conductivity are very high. It is in second place after silver in both of these indicators.
Comparison of conductivity of different types of steel
The characteristics of steel depend on its composition and temperature:
- For carbon alloys, the resistance is quite low: it is 0.13-0.2 μOhm/m. The higher the temperature, the greater the value;
- Low-alloy alloys have a higher resistance - 0.2-0.43 μOhm/m;
- High-alloy steels have high resistance - 0.3-0.86 μOhm/m;
- Due to the high chromium content, the resistance of chromium stainless alloys is 0.5-0.6 μOhm/m;
- Chromium-nickel austenitic steels are stainless and, thanks to nickel, have a high resistance of 0.7-0.9 μOhm/m.
Galvanized braiding is often made from steel.
Copper is in second place in terms of electrical conductivity: it perfectly passes electric current and is widely used in the manufacture of wires. Aluminum is also used no less often: it is weaker than copper, but cheaper and lighter.
Source
Connection of copper and aluminum wires
Recently, electrical equipment of increasingly higher power has begun to be used in everyday life and industry. During Soviet times, wiring was made mainly of cheap aluminum. Unfortunately, its performance characteristics no longer meet the new requirements. Therefore, today in everyday life and in industry very often aluminum wires are replaced with copper ones. The main advantage of the latter, in addition to refractoriness, is that during the oxidation process their conductive properties do not decrease.
Often when modernizing electrical networks, aluminum and copper wires have to be connected. This cannot be done directly. Actually, the electrical conductivity of aluminum and copper does not differ too much. But only for these metals themselves. The oxidizing films of aluminum and copper have different properties. Because of this, the conductivity at the junction is significantly reduced. The oxidation film of aluminum has much greater resistance than that of copper. Therefore, the connection of these two types of conductors must be made exclusively through special adapters. These could be, for example, clamps containing a paste that protects metals from the appearance of oxide. This adapter option is usually used when connecting wires outdoors. Branch compressors are more often used indoors. Their design includes a special plate that eliminates direct contact between aluminum and copper. If such conductors are not available at home, instead of twisting the wires directly, it is recommended to use a washer and nut as an intermediate “bridge”.
Application of copper conductors
Copper is not only a good conductor of electric current, but also a very ductile material. Thanks to this property, copper wiring fits better and is resistant to bending and stretching.
Copper is in great demand on the market. Many different products are made from this material:
- A huge variety of conductors;
- Auto parts (eg radiators);
- Clock mechanisms;
- Computer components;
- Parts of electrical and electronic devices.
The electrical resistivity of copper is one of the best among current-conducting materials, so many electrical industry products are created based on it. In addition, copper is easy to solder, so it is very common in amateur radio.
The high thermal conductivity of copper allows it to be used in cooling and heating devices, and its plasticity makes it possible to create the smallest parts and the thinnest conductors.
Physical properties
Thus, we found out what electrical conductivity copper has. This indicator may vary depending on the impurities contained in the metal. However, the demand for copper in industry is also determined by its other useful physical properties, information about which can be obtained from the table below.
- 5 - 9 grades
- Chemistry
- 5 points
thermal and electrical conductivity: COPPER. GLAND. MAGNESIUM. ZINC. ALUMINUM. SODIUM. URGENTLY
- Ask for more explanation
- Track
- Flag violation
Kisazayaryba 10/27/2012
What do you want to know?
Engineering Communication
The main advantages of copper water pipes are also durability and reliability. In addition, this metal is capable of imparting special unique properties to water, making it beneficial for the body. Copper pipes are also ideal for assembling gas pipelines and heating systems, mainly due to their corrosion resistance and ductility. In the event of an emergency increase in pressure, such lines can withstand a much greater load than steel ones. The only disadvantage of copper pipelines is their high cost.
Answer
Copper-thermal 382—390 W/(m K) electro58 100,000 cm/m
iron-thermal 92W/(m K) electrical 10,000,000 cm/m
magnesium-thermal 156 W/(m K) electro 22,700,000
Zinc-thermal 116 W/(m K) electrical 16,900,000
aluminum-thermal 237 W/(m K)electro(37 106 S/m)
All products used by humans are capable of transmitting and maintaining the temperature of the object or environment they touch. The ability of one body to transfer heat to another depends on the type of material through which the process takes place. The properties of metals allow heat to be transferred from one object to another, with certain changes depending on the structure and size of the metal structure. The thermal conductivity of metals is one of the parameters that determines their operational capabilities.
Copper and aluminum twist
It is strictly forbidden to twist aluminum cables with copper cables. These metals have different electrochemical properties. The resulting contact overheats, oxidizes and begins to burn. Hence all the ensuing consequences such as smoke and fire.
How to join copper and aluminum
For proper connection, you can use an intermediate conductor. Connect the copper and aluminum wire through an iron bolt with similar washers and nuts.
Bolted connection of copper and aluminum
Another common method is special Wago clamps with conductive lubricant. The connection will be significantly more expensive, but simpler, faster and more compact.
What is thermal conductivity and why is it needed?
The process of transferring the energy of atoms and molecules from hot objects to products with a cold temperature is carried out during the chaotic movement of moving particles. Such heat exchange depends on the state of aggregation of the metal through which the transmission passes. Depending on the chemical composition of the material, thermal conductivity will have different characteristics. This process is called thermal conductivity, it consists in the transfer of kinetic energy by atoms and molecules, which determines the heating of a metal product during the interaction of these particles, or is transferred from a warmer part to one that is less heated.
The ability to transfer or store thermal energy makes it possible to use the properties of metals to achieve the necessary technical goals in the operation of various components and assemblies of equipment used in the national economy. An example of such an application would be a soldering iron that heats up in the middle part and transfers heat to the edge of the working rod, which is used to solder the necessary elements. Knowing the properties of thermal conductivity, metals are used in all industries, using the required parameter for its intended purpose.
Chemical properties
According to these characteristics, copper, whose electrical and thermal conductivity is very high, occupies an intermediate position between the elements of the first triad of the eighth group and the alkali elements of the first group of the periodic table. Its main chemical properties include:
- tendency to form complexes;
- ability to produce colored compounds and insoluble sulfides.
The most characteristic of copper is the divalent state. It has practically no similarity with alkali metals. Its chemical activity is also low. In the presence of CO2 or moisture, a green carbonate film forms on the copper surface. All copper salts are toxic substances. In mono- and divalent states, this metal forms very stable complex compounds. Ammonia compounds are of greatest importance for industry.
The concept of thermal resistance and thermal conductivity coefficient
If thermal conductivity characterizes the ability of metals to transfer the temperature of bodies from one surface to another, then thermal resistance shows an inverse relationship, i.e. the ability of metals to prevent such transfer, in other words, to resist. Air has high thermal resistance. It is he who, most of all, prevents the transfer of heat between bodies.
The quantitative characteristic of the change in temperature of a unit area per unit of time by one degree (K) is called the thermal conductivity coefficient. The international system of units usually measures this parameter in W/m*deg. This characteristic is very important when choosing metal products that must transfer heat from one body to another.
Thermal conductivity coefficient of metals at temperature, °C
What does thermal conductivity depend on?
Studying the ability of heat transfer by metal products, it was revealed that thermal conductivity depends on:
- type of metal;
- chemical composition;
- porosity;
- sizes.
Metals have different crystal lattice structures, and this can change the thermal conductivity of the material. For example, in steel and aluminum, the structural features of microparticles affect differently the rate of transfer of thermal energy through them.
The thermal conductivity coefficient can have different values for the same metal when the exposure temperature changes. This is due to the fact that different metals have different melting degrees, which means that under other environmental parameters, the properties of the materials will also differ, and this will affect thermal conductivity.
What material for electrical wiring should you choose for your apartment?
In Soviet times, the use of aluminum electrical wiring in residential premises was common. This was due to the fact that in residential buildings there was no high load on the electrical network due to low power and a small number of electrical appliances. With the development of technology and the advent of a huge variety of powerful electrical appliances that are used at home, the requirements for the quality and materials for electrical cables have increased significantly. In modern realities, wiring made of aluminum material is practically not used, since according to the PUE, electrical wiring in residential premises must be made of copper !
Interesting fact! Not many people know, but a little earlier before aluminum wiring, in Stalin’s times, copper wiring was used in apartments.
Advantages and disadvantages of aluminum electrical wiring
The main advantages of aluminum electrical wiring are:
- Low mass: the density of aluminum is lower and, accordingly, its mass is lower. When laying simple networks with many cables but light loads, this will be a convenient advantage.
- Low price: aluminum is several times cheaper than copper, so products made from this material are also low in price.
- Resistance to oxidation: in the absence of contact with the environment, it lasts a long time and is not destroyed by oxidation.
Measurement methods
To measure the thermal conductivity of metals, two methods are used: stationary and non-stationary. The first is characterized by the achievement of a constant value of the changed temperature on the controlled surface, and the second - by a partial change in it.
Stationary measurement is carried out experimentally, requires a lot of time, as well as the use of the metal under study in the form of blanks of the correct shape, with flat surfaces. The sample is placed between the heated and cooled surface, and after touching the planes, the time during which the workpiece can increase the temperature of the cool support by one degree Kelvin is measured. When calculating thermal conductivity, the dimensions of the sample being studied must be taken into account.
Non-stationary research methods are used in rare cases due to the fact that the result is often biased. Nowadays, no one except scientists is involved in measuring the coefficient; everyone uses long-established experimental values for various materials. This is due to the constancy of this parameter while maintaining the chemical composition of the product.
Physical and mechanical properties of copper
Copper is a red-pink metal with a golden tint, occupying 29th place in the table of chemical elements and having a density of 8.93 kg/m3. The specific gravity of copper is 8.93 g/cm3, the boiling point is 2657, and the melting point is 1083 degrees Celsius.
This metal has high ductility, softness and ductility. Having high viscosity, it is excellent for forging. Copper is a fairly heavy and durable metal. In its pure form, it conducts heat and electricity well (second only to silver).
Thermal conductivity of steel, copper, aluminum, nickel and their alloys
Ordinary iron and non-ferrous metals have different structures of molecules and atoms. This allows them to differ from each other not only in mechanical properties, but also in thermal conductivity properties, which, in turn, affects the use of certain metals in various sectors of the economy.
Steel has a thermal conductivity coefficient at an ambient temperature of 0 degrees. (C) equal to 63, and when the degree increases to 600, it decreases to 21 W/m*degree. Aluminum, under the same conditions, on the contrary, will increase the value from 202 to 422 W/m*deg. Aluminum alloys will also increase thermal conductivity as the temperature increases. Only the value of the coefficient will be an order of magnitude lower, depending on the amount of impurities, and range from 100 to 180 units.
Copper, with a temperature change within the same limits, will reduce thermal conductivity from 393 to 354 W/m*deg. At the same time, copper-containing brass alloys will have the same properties as aluminum ones, and the thermal conductivity value will vary from 100 to 200 units, depending on the amount of zinc and other impurities in the brass alloy.
The thermal conductivity coefficient of pure nickel is considered low; it will change its value from 67 to 57 W/m*deg. Alloys containing nickel will also have a coefficient with a reduced value, which, due to the content of iron and zinc, ranges from 20 to 50 W/m*deg. And the presence of chromium will reduce the thermal conductivity in metals to 12 units, with a slight increase in this value when heated.
Brass
Alloys of copper and zinc, called brasses, are widely used in electrical engineering. Zinc dissolves in copper up to 39%.
In various brands of brass, the zinc content can reach up to 43%. Brasses containing up to 39% zinc have a single-phase solid solution structure and are called a-brasses. These brasses have the greatest ductility, so they are used to make parts by hot or cold rolling and drawing: sheets, strips, wire. Without heating, parts of complex configurations can be made from sheet brass using deep drawing and stamping.
Brasses with a zinc content of over 39% are called a+b-brasses or two-phase and are used mainly for shaped castings.
Two-phase brass is harder and more brittle and can only be pressed when hot.
The addition of tin, nickel and manganese to brass increases the mechanical properties and anti-corrosion resistance, and the addition of aluminum in composition with iron, nickel and manganese gives the brass, in addition to improving the mechanical properties and corrosion resistance, high hardness. However, the presence of aluminum in brasses makes soldering difficult, and soldering with soft solders becomes almost impossible.
· brass grades L68 and L63
Due to their high ductility, they are easily stamped and can be bent; they are easily soldered with all types of solders. In electrical engineering they are widely used for various live parts;
· brass grades LS59-1 and LMTs58-2
used for the manufacture of rotor (squirrel) cages of electric motors and for current-carrying parts made by cutting and hot stamping; soldered well with various solders;
· brass LA67-2.5
used for cast current-carrying parts of increased mechanical strength and hardness that do not require soldering with soft solders;
· brass LK80-3L and LS59-1L
widely used for cast current-carrying parts of electrical equipment, for brush holders and for casting rotors of asynchronous motors. They accept soldering well with various solders.