Visual definition
With a high content of zinc or tin, copper alloys are quite easy to distinguish by color . In brass, the color shade due to the presence of zinc shifts from pink-red copper to golden-yellow tones and pure gold.
Bronze with a high amount of tin acquires a light silvery color, and with a small amount of alloying additive it remains closer to copper with its red tones. But a visual comparison of brass and bronze is almost impossible when the amount of impurities is in the range of 15-35%. With this composition, the color shades of both alloys are completely identical.
In practice, compounds with high additive content are quite rare. Therefore, we can say that brass has a golden-yellow hue, and bronze is reddish, or the alloys are very similar to each other.
How to distinguish copper by eye
Visual examination of a non-ferrous metal or alloy is not the most effective method for identifying copper. It is sufficient to distinguish copper from aluminum, nickel and zinc.
The natural color of copper is red-pink, without distortion it is easy to determine in natural light. This is important to consider because artificial light can give the metal a yellowish-green tone.
During visual examination, it is necessary to remove the oxide from the metal. This is easy to do by treating the surface of the product with a file or cutting it.
It is difficult to distinguish copper from bronze and brass, as well as from copper-plated aluminum. At receiving points, copper is valued more expensive than bronze, aluminum and brass. For this reason, it is necessary to be able to accurately identify this non-ferrous metal in various ways.
Exposure to high temperatures
Heating to 600˚C allows you to determine the type of copper alloy. The alloying impurities included in its composition react to high temperatures in different ways. Bronze simply heats up, and if you try to bend its sample, it will most likely break. In brass, zinc oxidation when heated causes a white coating to appear on the surface. The metal becomes more ductile and does not break when deformed.
To carry out such a test, you need a fairly powerful gas burner, since the flame of a gas stove, much less a lighter, will not be enough.
Magnet check
Electromagnetic waves emitted by the substance are responsible for the magnetic properties of objects. When interacting with a magnet, some metals are attracted, but some do not react, since there is no electromagnetic radiation. Cuprum also belongs to these. This metal is diamagnetic, and therefore will not react to a magnet. Moreover, the copper field is repelled by the magnet. This unique property has led to the use of metal in electrical products, since under the influence of current it creates the necessary field for the movement of electronic particles. If a magnet is attracted to the sample, it means that it is an alloy in which there is no more than half of the required metal.
Expert opinion
Sidorenko Alexander
Antiques appraiser, numismatist
But sometimes there are samples that also do not have magnetic properties, although they do not consist of pure copper. This happens when there is aluminum underneath the copper coating. It is also diamagnetic, so aluminum products will not be attracted to a magnet.
An object made of pure cuprum may become magnetic over time if it oxidizes. As a result, a film is created on the surface that has high ferromagnetic properties.
Use of chemical reagents
The use of any reagents is a fairly effective, but destructive way to determine the type of copper alloy . It is carried out in several stages:
- it is necessary to remove chips from the surface of the sample or separate a small piece of thickness;
- prepare a solution of nitric acid (HNO3) in a 1:1 ratio with water;
- place the prepared shavings in the reagent;
- Heat the container with the acid solution to boiling temperature and maintain it until the chips are completely dissolved.
- The presence of brass will leave the solution clear without changing color .
- Bronze produces a white precipitate as a result of the reaction of tin with acid.
general information
Copper (cuprum) is a metal that has a golden-reddish color and is characterized by high thermal and electrical conductivity. Another distinctive quality of the element is its high ductility. Nuggets are found less and less often in nature; they are most often mined from ore.
Is copper magnetic or not?
When determining the authenticity and purity of a sample, you can turn to an expert, but determining a chemical element in a laboratory is quite expensive. Therefore, you need to focus on several home methods.
First of all, we take a closer look at the color of the product. Since this element tends to oxidize, it is necessary to evaluate the cut or cut of the object. For accuracy, take a sample and compare the color. It should be golden-reddish. Gold has similar colors, as do osmium and cesium.
If copper wire is set on fire, it will not burn, but will first lose its shine and then change color to dark.
You can treat the sample with nitric acid - it should acquire a greenish-blue tint.
Determination of the physical density of an alloy
The difference in the specific gravity of brass and bronze differs slightly, and in a certain range of values it even completely coincides. Depending on the quantitative content of the alloying additive, the density of the compounds is:
- brass 8.4 - 8.7 g/cm3;
- bronze 7.4 - 8.9 g/cm3;
From these indicators it is clear that determining the ratio of the weight of a sample to its volume allows one to determine only bronze with a high content of light tin. Other grades of these alloys may have the same density.
Determination by color
The easiest way to determine whether a product is copper or brass is by its color. For accuracy, it is recommended to thoroughly clean the metal surface from dirt and oxide film. As mentioned earlier, copper has a reddish tint, sometimes brownish or pink.
If the product under study has a yellowish color, reminiscent of gold, then this is most likely brass. And the more pronounced the yellowness, the greater the proportion of zinc in the alloy.
You can determine the metal by color by comparison with a known product. In everyday life, as a copper sample, you can use an electrical wire, cleared of insulation and protective varnish. Brass can be seen on the plugs of electrical appliances - their pins are made from this alloy.
Reserves, production
The world's proven reserves of copper ore amount to billions of tons. Scientists hope that the earth's bowels conceal another 3 billion tons. The richest mines, Chuquicamata and El Teniente, are located in Chile, the largest copper producer (27% of world production in 2022).
Large deposits have been found in the USA, Canada, Iran, Kazakhstan, and southern Africa. Russia contains 3% of the world's reserves. Copper deposits have been found in the Urals, Transbaikalia, and Krasnoyarsk Territory.
It is desirable that the ore contains at least 0.5-1% pure copper. It is mined in mines and by open-pit mining (for deposits located at a depth of 400-500 m). Global production in 2022 amounted to 20.6 million tons, of which a quarter was of Chilean origin.
How to determine copper wire or aluminum
Advantages of copper cable. Why do many people prefer cables with copper filling?
Copper cable has better conductivity compared to aluminum. With the same cross-sectional area of the cable core, copper can withstand loads significantly greater than aluminum.
For example, with a cross-sectional area of 10 mm2, an aluminum conductor can withstand an electric current of up to 50A, and a copper conductor of the same cross-section can withstand a current of up to 70A.
That is, if it is necessary to replace an aluminum cable along a ready-made main line and the thickness of the cable is limited, and the expected load has increased, then laying a copper cable instead of aluminum will allow, with the same cable dimensions, to increase the permissible load.
Copper cable has better conductivity compared to aluminum. With the same cross-sectional area of the cable core, copper can withstand loads significantly greater than aluminum.
For example, with a cross-sectional area of 10 mm2, an aluminum conductor can withstand an electric current of up to 50A, and a copper conductor of the same cross-section can withstand a current of up to 70A.
That is, if it is necessary to replace an aluminum cable along a ready-made main line and the thickness of the cable is limited, and the expected load has increased, then laying a copper cable instead of aluminum will allow, with the same cable dimensions, to increase the permissible load.
Copper cable has greater chemical resistance compared to aluminum. Copper is a noble (inert) metal and does not react chemically with most substances. And aluminum is exposed to chemical attack, as a result of which it is destroyed.
Copper cable has greater mechanical strength compared to aluminum. This can be observed at the connection points of aluminum cables in home wiring. In the area of the terminals, the aluminum core is always very crushed and often destroyed, which never happens with a copper core.
Advantages of aluminum cable.
Aluminum cable is suitable for temporary wiring. Due to its low cost (low cost is at least three times cheaper than a copper cable), you can save a lot on this type of cable.
First of all, it is, of course, lightweight. This is an indisputable advantage: it is more convenient to roll out a coil or coil with a lightweight cable, and when it comes to installing power lines, then lightness becomes the most valuable quality.
But in addition to the advantages, there are also disadvantages of aluminum cable.
Aluminum as a conductor, compared to copper, has a higher electrical resistivity - 0.0271 Ohm x sq. mm/m versus 0.0175 Ohm x sq. Mmm. The difference is almost double!
It is the high resistivity that negates the advantage of aluminum being lightweight. It turns out that in order to ensure the same conductivity, we will have to take a much more powerful, and therefore heavier, aluminum conductor than if we used copper.
Everyone knows very well that aluminum is a corrosion-resistant metal. But from the chemistry course we know that this is not entirely true. Aluminum itself oxidizes in air very quickly. But the resulting thin film of oxide protects it from further chemical destruction.
But the protective film already has slightly different properties than the metal itself. In particular, it is no longer such a good conductor. This means that increased contact resistance may form at the point of electrical contact with the aluminum oxide film. And this leads to heating of the contact, which in turn leads to an even greater increase in electrical resistance.
This is such a vicious circle. The result is melted contacts, an open circuit or unreliable power supply.
You have to look for the problematic contact, tighten it, or change the clamps, and aluminum subjected to prolonged heating, which already does not have much ductility, can break off from any careless movement. Then a cable replacement will be required, which is not always technologically possible.
However, the use of a particular cable also depends on the purpose for which it is used. Each electrical appliance has its own power, which will directly determine the cross-section of the cable, and therefore its filling.
In the cable abbreviation, to denote an aluminum core, the letter A is at the beginning. That is, A is added to the already familiar rulers, and the result is AVVG, AVVGng, AVVGng(A)-LS, AVBbShv, AVBShv, respectively.
If you have decided on the type of cable that is right for you, be sure to check the quality of the product before making a purchase. A cable made of any metal must be stored in appropriate conditions and have all the necessary technical documentation.
Advantages of aluminum wiring
The fact that aluminum, not being a standard for electrical wiring, is preferable in its quality in residential buildings is due precisely to its advantages over other metals.
Determination using chemistry
This method is one of the simplest and most accessible, and at the same time quite accurate. To determine the composition of the metal, you will need a solution of hydrochloric acid. Such liquids are often used to clean contacts when soldering in radio electronics. Accordingly, acid can be bought at any radio store. And it's inexpensive.
Without going into details and without resorting to chemical formulas, the essence of the test is as follows. A few drops of acid must be applied to the surface of the metal being tested. If it is copper, then it will simply be cleaned and acquire its natural reddish or pinkish tint. If we have brass in front of us, then a chemical reaction will take place on its surface with the release of a white substance - zinc oxide.
Interesting Facts
Since the use of copper is very wide, there are, accordingly, a lot of interesting facts related to copper. It’s worth starting with the fact that the price of pure copper on the world market is not so low. In 2014, 1 ton of copper on the world market was valued at 7,000 US dollars. Due to such a high price, the number of thefts of copper items has increased. For example, in Germany, the railway company Deutsche Bahn AG suffered losses of 14 million euros due to the theft of copper grounding cables.
Another interesting point is that the first mirrors invented by man were made of copper. The copper was rubbed (polished) until a reflection on the surface of the copper was visible. Also in the field of application, it is worth noting that most of the coins produced around the world contain copper. Another interesting fact is that copper, like iron and aluminum, can be recycled without losing its properties.
The biological component of copper can also be noted. In large quantities it is toxic, but in small quantities it is an integral part of the existence of the human body. In various states, the human body contains about 150 mg of copper. The normal daily intake of copper for a person weighing 75 kg is 2 mg.
Copper Applications
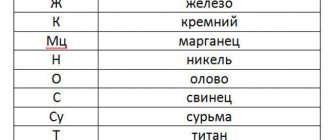
Due to their valuable qualities, copper and copper alloys are used in the electrical and electrical engineering industries, in radio electronics and instrument making. There are alloys of copper with metals such as zinc, tin, aluminum, nickel, titanium, silver, and gold. Less commonly used are alloys with non-metals: phosphorus, sulfur, oxygen. There are two groups of copper alloys: brass (alloys with zinc) and bronze (alloys with other elements).
Copper is highly environmentally friendly, which allows its use in the construction of residential buildings. For example, a copper roof, due to its anti-corrosion properties, can last more than a hundred years without special care or painting.
Copper in alloys with gold is used in jewelry. This alloy increases the strength of the product, increases resistance to deformation and abrasion.
Copper compounds are characterized by high biological activity. In plants, copper takes part in the synthesis of chlorophyll. Therefore, it can be seen in the composition of mineral fertilizers. A lack of copper in the human body can cause deterioration in blood composition. It is found in many food products. For example, this metal is found in milk. However, it is important to remember that excess copper compounds can cause poisoning. This is why you should not cook food in copper cookware. During boiling, large amounts of copper can leach into food. If the dishes inside are covered with a layer of tin, then there is no danger of poisoning.
In medicine, copper is used as an antiseptic and astringent. It is a component of eye drops for conjunctivitis and solutions for burns.