Cyanidation of steel is one of the ways to improve the physical and chemical properties of the metal. The use of the method is necessary when it is necessary to increase the strength, hardness, corrosion resistance, wear resistance of the surface layer of steel, and make it more resistant to natural aging.
Nitrocarburization strengthens steel by exposing it to carbon and nitrogen, or rather, introducing these molecules into the crystal lattice of the surface layer. This entire process occurs under the influence of high temperatures in an environment of sodium cyanide salts, the oxidation of which leads to the release of carbon and nitrogen.
How deeply the cementitious substances penetrate into the metal structure and what degree of concentration is formed depends on the selected temperature of the operation and the time interval of exposure. Nitrocarburizing and cyanidation of steel are operations that pursue the same goal, but take place in different environments.
The purpose of steel cyanidation and the essence of technology
The primary goal of cyanidation is to strengthen the surface layer of steel of various parts, giving it a higher endurance limit, since this layer is subject to the greatest loads during operation of mechanisms and structures. Saturation of the surface layer of the metal with carbon and nitrogen is usually used due to their rapid penetration when they interact simultaneously. The following types of metal can be processed using cyanidation:
- any stainless steel;
- alloyed steel alloys or those without the presence of alloying components, steels with an average carbon concentration;
- steel for structural purposes, where there is little carbon present.
The chemical-thermal cyanidation method adheres to the following technology:
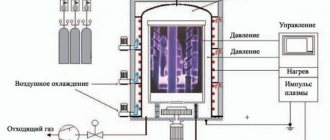
- The part is placed in a working bath with molten cyanide salt of the composition 15% Na₂CO₃, 60% NaCl and 25% NaCN.
- Next, the working medium is heated to a temperature from 930 to 530 degrees Celsius (depending on the selected processing mode).
- Carbon monoxide and nitrogen released from the salt saturate the metal for several hours.
The essence of the process by which carbon and nitrogen can penetrate into a layer of steel is diffusion. During the technology stages listed above, the process goes through two main stages, separated by time periods:
- The initial period of nitrocarburization lasting from one to three hours, characterized by the introduction of nitrogen and carbon atoms into the crystal lattice of the metal.
- The final period when nitrogen atoms that have previously penetrated and saturated the steel begin to desorb (leave the surface, again acquiring the state of a gas), while carbon continues to saturate the metal until the effect of temperature and working environment ends.
Links[edit]
- Roubo, Andreas; Kellens, Raf; Reddy, Jay; Steyer, Norbert; Hasenpusch, Wolfgang (2006). "Alkali metal cyanides". Ullman Encyclopedia of Industrial Chemistry
. DOI: 10.1002/14356007.i01_i01. ISBN 978-3527306732. - Barrick Gold - Cyanide Facts Archived 2010-09-20 at the Wayback Machine
- ^a b Gray, JA; McLachlan, J. (June 1933). "The History of the Introduction of the MacArthur-Forrest Cyanide Process to the Witwatersrand Goldfields". Journal of the South African Institute of Mining and Metallurgy
.
33
(12): 375–397. HDL: 10520/AJA0038223X_5033. - us US403202 , MacArthur, John Stewart; William Forrest and Robert Forrest Robert, "The Process of Obtaining Gold and Silver from Ores," issued May 14, 1889.
- "Gold Recovery Methods II". 2013-05-14.
- ^ ab Habashi, Fathi's latest achievements in gold metallurgy. Archived March 30, 2008, at the Wayback Machine.
- Alumni quarterly and fortnightly notes. University of Illinois. January 1, 1921. Retrieved May 1, 2016.
- "Mercur, UT". Retrieved May 1, 2016.
- Adams, Mike D. (2005-12-02). Advances in gold ore processing
. Elsevier. pp. XXXVII – XLII. ISBN 978-0-444-51730-2. ISSN 0167-4528. - Greenwood, New York; & Earnshaw, A. (1997). Chemistry of the Elements (2nd ed.), Oxford: Butterworth-Heinemann. ISBN 0-7506-3365-4.
- (Internet Archive) Technical Bulletin, https://web.archive.org/web/20091023235047/https://www.multimix.com.au/DOCUMENTS/Technical%20Bulletin1.PDF
- ^ ab UNEP/OCHA Environment Division "UN Assessment Mission - Baia Mare Cyanide Spill, March 2000"
- Maprani, Antu S.; Al, Tom A.; MacQuarrie, Kerry T.; Dalziel, John A.; Shaw, Sean A.; Yates, Philip A. (2005). “Determining Mercury Evasion in a Contaminated Upstream.” Environmental Science and Technology
.
39
(6):1679–1687. Bibcode: 2005EnST…39.1679M. DOI: 10.1021/es048962j. PMID 15819225. - ↑
Al, Tom A.;
Leybourne, Matthew I.; Maprani, Antu C.; MacQuarrie, Kerry T.; Dalziel, John A.; Fox, Don; Yates, Philip A. (2006). "The influence of acid-sulfate weathering and cyanide-bearing gold tailings on the transport and fate of mercury and other metals in Gossan Creek: Murray Brook Mine, New Brunswick, Canada." Applied Geochemistry
.
21
(11): 1969–1985. Bibcode: 2006ApGC...21.1969A. DOI: 10.1016/j.apgeochem.2006.08.013. - "Long-term persistence of cyanide species in the mine waste environment", B. Yarar, Colorado School of Mines, Tailings and Mine Waste '02, Swets & Zeitlinger ISBN 90-5809-353-0, p. 197 (Google Books)
- ↑
BBC News BBC: "Cyanide seeps into PNG rivers" 23 March 2000 - Wilson, T.E. La politica es la politica: "After the Cyanide Spill, Can First Majestic Clean Up Its Effects?" April 21, 2018.
- La Broix, S.R.; Linge, H. G.; Walker, G. S. (1994). "Review of gold mining from ores." Mineral Engineering
.
7
(10): 1213–1241. DOI: 10.1016/0892-6875 (94) 90114-7. - ↑
Citizens' initiative to ban cyanide mining in Montana, USA. Archived October 21, 2007, at the Wayback Machine. - ↑
Senate Bill 160 of 2001 on the use of cyanide in mining. - "Czech Senate bans cyanide use in gold mining". Nl.newsbank.com. 2000-08-10. Retrieved January 3, 2013.
- Zöld siker: törvényi tilalom a cianidos bányászatra! Archived July 21, 2011, at the Wayback Machine.
- International Mining - European Commission rejects proposed ban on cyanide in mining, July 2010.
- Council Directive 96/82/EC of 9 December 1996 on the control of the hazards of major accidents involving hazardous substances. For changes, see summary version.
- Council Directive 82/501/EEC of 24 June 1982 on the hazards of major accidents in certain industrial activities. Not valid.
- Council Directive 80/68/EEC of 17 December 1979 on the protection of groundwater against pollution caused by certain hazardous substances. Not valid.
- Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy (Water Framework Directive). For changes, see summary version.
- Directive 2006/21/EC of the European Parliament and of the Council of 15 March 2006 on the management of waste from extractive industries. For changes, see summary version.
- ICMI www.cyanidecode.org International Cyanide Code for the Production, Transport and Use of Cyanide in Gold Production
Related methods
A similar method is considered to be soft nitriding. It is performed at a temperature of about 590°C. This treatment is used to increase the wear resistance and endurance limit of medium-carbon steels.
Also, in terms of technology, the treatment in question is close to cementation. Compared to cyanidation, cyanidation stands out in that the layer formed has better wear resistance and corrosion resistance, greater hardness, and fatigue strength. Moreover, due to lower temperatures and duration of the process, grain growth does not occur. In view of this, immediately after cyanidation is completed, hardening is performed, which gives the surface greater hardness. Finally, the high-temperature cyanidation process of steel takes less time than carburizing.
If you find an error, please select a piece of text and press Ctrl+Enter.
Advantages, disadvantages
When choosing a processing method, it is necessary to take into account the thickness of the products, since thin objects subjected to cyanidation may be more fragile than parts processed using conventional carburizing technology. This is a disadvantage of the technology under consideration. In addition, as a result of such processing, the properties of not the entire material change, but only its surface layer up to 1.6 mm thick. Finally, during cyanidation, constant monitoring of the degree of carburization and nitriding of the working environment is necessary.
Cyanidation of steel
This process is somewhat different from carburization and consists in the fact that the surface layer of the steel element is saturated not only with carbon, but also with nitrogen. In industry, high- and low-temperature cyanidation is used, while carburization does not allow several types of operations.
High temperature cyanidation
The main objective of this process is to make the part harder and more wear-resistant.
The manipulation is carried out in baths that are filled with neutral salts: BaCl2, NaCl, Na2CO3 and some others. The role of carburizers is performed by KCN and NaCN salts, the active ingredient of which is cyanogen. It contributes to the fact that the steel part is saturated with nitrogen and carbon. The process is carried out at temperatures up to 900 °C. In order for the cyanidated layer to become as durable as possible, the parts are hardened either in oil or in water for one and a half hours. To prevent the amount of cyanide from decreasing (it gradually burns out), small portions of cyanide salts are added to the bath.
Low temperature cyanidation
This process is appropriate if the part must meet the criteria of increased strength and wear resistance. The temperature required to achieve these goals ranges from 550 to 570 °C (high-speed steel) and 510–520 °C (high-chromium steel).
The procedure is carried out in a salt bath, the contents of which are equal parts of NaCN and KCN. The depth of the resulting layer is from 0.01 mm (with a cyanidation duration of 10 minutes) to 0.06 mm (with a process duration of up to 60 minutes).
Legislation[edit]
See also: Ban on cyanidation of gold
The American states of Montana [19] and Wisconsin, [20] the Czech Republic, [21] Hungary, [22] have banned cyanide mining. The European Commission rejected the proposal for such a ban, noting that existing rules (see below) provide adequate environmental and health protection. [23] Several attempts to ban cyanidation of gold in Romania were rejected by the Romanian parliament. There are currently protests in Romania calling for a ban on the use of cyanide in mining (see 2013 Romanian protests against the Roșia Montană project).
In the EU, the industrial use of hazardous chemicals is regulated by the so-called Seveso II Directive (Directive 96/82/EC,[24] which replaced the original Seveso Directive (82/501/EEC[25], introduced after the 1976 dioxin disaster. "Free cyanide and any compound capable of releasing free cyanide in solution" are further controlled by inclusion in Schedule I of the Groundwater Directive (Directive 80/68/EEC) [26], which prohibits any releases the amount of which is likely to cause a deterioration in the quality of groundwater at that time or in the future. The Groundwater Directive was largely replaced in 2000 by the Water Framework Directive (2000/60/EC) [27].
In response to the Baia Mare cyanide spill in 2000, the European Parliament and Council adopted Directive 2006/21/EC on the management of waste from extractive industries. [28] Article 13(6) requires that "the concentration of weakly acidic dissociable cyanide in a pond shall be reduced to the lowest possible level using the best available technology", and almost all mines commenced after 1 May 2008 may not discharge waste containing more than 10 ppm Cyanide WAD, mines constructed or permitted before this date are initially allowed no more than 50 ppm, decreasing to 25 ppm in 2013 and 10 ppm by 2022.
Under Article 14, companies must also provide financial guarantees to ensure cleanup after completion of the mine. This may in particular impact smaller companies wishing to build gold mines in the EU, as they are less likely to have the financial capacity to provide such guarantees.
The industry has developed a voluntary Cyanide Code
[29], which aims to reduce environmental impacts through third-party audits of a company's cyanide management activities.
Anodizing of various metals
Stainless steel
The most difficult object to anodize due to its chemical inertness. To obtain an oxidized surface, the stainless steel is first subjected to a nickel plating procedure. Although active development of special diffusion pastes is currently underway, on which the oxide will form without a nickel “cushion”.
Copper
It is difficult to oxidize, and where this is required, expensive salts are used as additives to electrolytes or non-environmentally friendly phosphate or oxalate solutions are used. In practice, this process is used extremely rarely.
Titanium
Metal products made of titanium undergo a mandatory oxidation procedure, due to the fact that the application of an oxide film increases the wear resistance of the top layer of titanium products by 15-28%. It also additionally gives the products a decorative effect, radically changing the color. Titanium is very undemanding when it comes to the composition of acids for electrolytic reactions—almost any will do.
Silver
To create an oxide film on silver, liver of sulfur is used - an alloy of powdered sulfur with potash under strong heating without the presence of water. However, this method of applying oxide films is also used for bronze, where the resulting film is called artificial patina. On silver, treatment with this reagent can produce blue and violet colors. But without changing the properties of silver as a metal.
Aluminum anodizing
Oxidation of this metal provides the widest possibilities with the widest scope of application. There are many ways to form oxides on the surface of this metal, more than half of them involve obtaining brightly colored surfaces.
Story
In 1783, Karl Wilhelm Scheele discovered that gold dissolves in aqueous solutions of cyanide. Thanks to the work of Bagration (1844), Elsner (1846) and Faraday (1847), it was determined that for each gold atom one cyanide ion was required, that is, the stoichiometry of the soluble compound.
Industrial process
John Stewart MacArthur developed the cyanide process to extract gold in 1887.
The expansion of gold mining in the Rand of South Africa began to slow in the 1880s, as new deposits were discovered, usually pyrite ores. Gold could not be extracted from this compound by any of the chemical processes or technologies then available. In 1887, John Stuart MacArthur, working in collaboration with brothers Robert and William Forrest for the Tennant Company in Glasgow, Scotland, developed the MacArthur–Forrest process for extracting gold from gold ores. Several patents were issued that year. By suspending crushed ore in a cyanide solution, separation of up to 96% pure gold was achieved. The process was first used at Rand in 1890 and, despite operational shortcomings, led to a boom in investment as larger gold mines were opened.
By 1891, Nebraska pharmacist Gilbert S. Peyton had perfected the process at his Mercure Mine in Utah, "the first mining operation in the United States to achieve commercial success with the cyanide process on gold ores." In 1896, Bodlender confirmed that oxygen was needed for this process, which MacArthur had doubted, and discovered that hydrogen peroxide was formed as an intermediate product. Around 1900, American metallurgist Charles Washington Merrill (1869-1956) and his engineer Thomas Bennett Crowe improved the purification of cyanide leachate using vacuum and zinc dust. Their process is the Merrill–Crowe process.
Advantages of nitrocarburization over cementation
The nitrocarburizing process is the safest and most advanced method of strengthening steel with carbon and nitrogen. Compared to conventional cementation, it has a number of advantages:
- surface diffusion occurs faster;
- no preparation required;
- During nitrocarburization, the metal is not subjected to severe overheating, and, as a result, the crystal lattice does not change;
- workpieces are less susceptible to deformation;
- after processing, subsequent hardening and tempering are carried out with better quality;
- Nitrocarburization is the cheapest way to strengthen steel, which is why it is widely used in mechanical engineering.
How does cyanidation work?
The most popular cyanidation option is low-temperature treatment. The method is applicable for parts and tools made of high-speed steels. The procedure itself is performed at a temperature of only 550-570 degrees Celsius in salt baths.
Cyanide bath options:
- 50% potassium cyanide and 50% sodium cyanide. The average melting point of the mixture is about 490°C.
- 96-98% sodium cyanide and 4-2% soda. The mixture melts at a temperature of 550°C.
- 60% sodium cyanide and 40% soda. The melting point of the composition is about 440°C.
The first two mixtures are quite thick. The latter mixture, which includes sodium and soda, is distinguished by a more liquid form and the absence of salts adhering to the metal surface. Due to these factors, the latter mixture is used much more often than the other two.
Cyanidation of steel can be carried out only after heat treatment of the metal and its final sharpening. During steel processing, parts are immersed in a container with salts in a liquid state. For this purpose, special hooks or wire are most often used, the size of which depends on the volume and weight of the part. The holding time of the steel part is from 5 to 30 minutes. After lifting the steel part from the container, a cyanidated layer is formed on the metal, the thickness of which is 0.02-0.07 mm. The upper part of the layer has a fairly small thickness, so it wears off very quickly during use. The internal part has greater strength characteristics, as well as increased wear resistance.
Efficiency
The effectiveness of cyanidation, although confirmed, does not have a single indicator. It all depends not only on the quality of processing, but also on the method of regrinding the parts, as well as their wear pattern. The greatest efficiency of cyanidation is observed when processing the following instruments:
- thread and hob cutters;
- shaped cutters and taps;
- Dolbyakov.
During the pointing process, grinding is performed exclusively along the front surface. High efficiency of surface treatment is observed in drills and countersinks due to the preservation of the cyanide layer on the front surfaces and additional cutting blades. Since the layer is completely removed when regrinding spline cutters and cutting tools, the products must be reprocessed after the point.
It is also worth considering that cyanidation can increase the fragility of the teeth of the part. Since the material wears out not only along the back wall, in the future the layer can act as an abrasive, which will lead to a premature change in the durability of the part. Before performing cyanidation, you need to carefully consider where the part will be located.
Purpose of the process
Normalization is designed to change the microstructure of steel; it does the following:
- reduces internal stress;
- through recrystallization, it refines the coarse-grained structure of welds, castings or forgings.
The goals of normalization can be completely different. Using this process, the hardness of steel can be increased or decreased, the same applies to the strength of the material and its toughness. It all depends on the mechanical and thermal characteristics of the steel. Using this technology, it is possible to both reduce residual stresses and improve the degree of machinability of steel using one or another method.
Steel castings are subjected to this treatment for the following purposes:
- to homogenize their structure;
- to increase susceptibility to heat hardening;
- to reduce residual stresses.
Products obtained by forming are subjected to normalization after forging and rolling in order to reduce the heterogeneity of the structure and its banding.
Normalization together with tempering is needed to replace the hardening of products with complex shapes or with sharp changes in cross-section. This will prevent defects.
This technology is also used to improve the structure of the product before hardening, increase its machinability through cutting, eliminate the secondary cement network in hypereutectoid steel, and also prepare the steel for final heat treatment.
Classification of the environment in which steel carburization takes place
Enriching steel with carbon and changing the atomic lattice of the metal can be carried out in different environments:
- hard;
- gaseous;
- liquid;
- electrolytic solution.
It is also possible to carry out cementation using pastes.
Each processing method requires separate consideration, as it has its own characteristics.
Cementation using solid media
For processing to be successful, it is necessary to use a solid carburizer. In production, a mixture of charcoal obtained from oak and birch is used for this. Additionally, a carbonic acid salt is added to the coal, which is saturated with calcium or other alkali metals. In order for the carbon to come out faster and saturate the steel, the pre-prepared mixture is crushed to a fine fraction. It is sifted through several sieves so that the output is identical grains measuring 10 mm in size.
The working process:
- When the mixture is prepared, it is placed in boxes.
- Later, blanks are placed in them. The boxes are sealed on all sides and heated to 800 degrees.
- The temperature slowly rises to 950 degrees.
The duration of processing will depend on the thickness of the carbon layer required to be obtained at the output.
Charcoal (Photo: Instagram / coalbaltic)
Cementation in a gas environment
Processing in a gas environment is used in the manufacture of engines. The steel is enriched with carbon only 2 mm deep. Any mixtures enriched with carbon are used as gases.
Processing stages:
- The blanks are placed in a sealed oven. It heats up to 950 degrees.
- Gradually, gas saturated with carbon begins to flow into the furnace.
- The workpiece is aged for 12 hours.
A layer of 1.2 mm grows on the surface of the steel. If it is necessary to speed up processing, the temperature can be raised above 1000 degrees. Thanks to this, the process is reduced by 4 hours.
Cementation in a liquid medium
The words “liquid medium” mean molten salts.
Processing stages:
- Molten salt baths are heated to 850 degrees.
- The workpieces are lowered into them and left for a long time.
To obtain cemented steel in a liquid base, the maximum layer thickness should be 0.5 mm. To get this result, you need to wait 3 hours.
Molten salt baths
Cementation in vacuum
To speed up the steel processing process, a vacuum carburizing method is used. Processing stages:
- Initially, the blanks are laid out in the oven. It is sealed.
- A vacuum is created inside.
- The oven begins to heat up to a certain temperature.
- The average exposure time is 60 minutes.
- Next, the chamber is filled with hydrocarbon gas. The upper layers are enriched in carbon.
- A vacuum is created again in the oven.
A carburized layer of the required thickness is obtained only after three stages of creating a vacuum and supplying hydrocarbon under pressure. The workpieces are cooled in a furnace using inert gases.
Cementation with pastes
One of the popular methods of cementation is processing using pastes. They consist of charcoal dust. Pastes are applied to the workpiece. The composition is applied in such layers that it is 8 times larger than the required thickness of the carbon layer. Next, the workpieces are placed in an induction furnace and heated to a temperature of 1000–1100 degrees.
Cementation in electrolytic solution
The processing process involves placing the workpieces in an electrolyte solution. Initially, it heats up to 450–1050 degrees. Next, a voltage of 150–300 volts is applied to the solution. The metal is enriched with carbon.
Processed products (Photo: Instagram / zubixdetal)
Alternatives to cyanide[edit]
Although cyanide is cheap, effective and biodegradable, its high toxicity has led to new methods of gold recovery using less toxic reagents. Other extractants have been tested including thiosulfate (S2O32-), thiourea (SC(NH2)2), iodine/iodide, ammonia, liquid mercury and alpha-cyclodextrin. Challenges include reagent costs and gold recovery efficiency. Thiourea is used on an industrial scale for ores containing stibnite. [18]
Nitriding of steel
When nitriding, the surface layer of a steel part is saturated with oxygen. This method received industrial application almost 100 years ago, in the 20s of the 20th century. Nitriding a part is an excellent way to increase not only the hardness of the product, but also its corrosion resistance.
Nitriding of steel is carried out by immersing the part in furnaces, which are hermetically sealed. Ammonia is supplied there, which, when heated, breaks down into nitrogen and hydrogen. During this reaction, nitrogen atoms are absorbed by the steel surface layer and penetrate into the part.
It is difficult to say how deep and durable the layer subject to nitriding will be. This factor depends on many details:
- temperature at which nitriding was carried out;
- duration of part processing;
- composition of steel that has been nitrided.
Chemical-thermal treatment method
The described procedure does not allow achieving several goals simultaneously, unlike cementation. There are two types of nitriding.
Increasing the strength of the surface layer of a steel part. The process temperature is up to 560 °C, the average layer thickness is 0.5 mm. The duration of the operation can reach one day.
Increased corrosion resistance. The optimal temperature is from 650 to 700 °C. Anti-corrosion nitriding can continue for up to 10 hours. The thickness of the layer formed in the process is 0.3 mm.
The steel nitriding process can only be carried out on fully finished products that have gone through the stages of heat and mechanical treatment. The structure of sorbitol inside the product is completely preserved, which increases the strength and toughness of the part.
3.5.g Galvanizing
Galvanizing is the process of diffusion saturation of the surface of a part with zinc.
Chemical-thermal galvanizing methods include hot galvanizing or immersion galvanizing, galvanizing in zinc powder - sherardization, galvanizing in zinc vapor. In addition to these methods, electrolytic galvanizing, spray metallization and application of zinc-containing paints are used. Galvanizing is a process that dramatically increases corrosion resistance. Increasing corrosion resistance when galvanizing steel parts is achieved through two chemical processes: zinc, being an electropositive metal in relation to iron, inhibits corrosion of the surface of the part. Under the influence of atmospheric moisture, a layer of zinc carbonates and oxides is formed on the galvanized surface of a steel part, which also has a protective effect. The galvanizing temperature depends on the method of operation. Thus, when galvanizing in powders, the process temperature ranges from 370–430 °C, when galvanizing by immersion - 430–470 °C. The range of holding times for galvanizing is also wide. If, when galvanizing in powder mixtures, a layer thickness of about 0.1 mm is achieved on average in 10 hours, then when galvanizing by immersion, a layer thickness of 0.3 mm is obtained in the first 10 seconds of the process. Galvanizing in zinc vapor is carried out in a reducing hydrogen environment at temperatures of 850–880 °C and a pressure of about 80 mm of water column. The time of such a process is quite long and usually amounts to tens of hours. The thickness of the resulting layers usually does not exceed 0.1–0.2 mm.
Depending on the saturation mode, an η-phase (solid solution of iron in zinc) can form in the diffusion layer on the surface of iron, then a layer of intermetallic phases FeZn13, FeZn7, Fe3Zn10, and closer to the core - a solid solution of zinc in iron.
To increase the corrosion resistance of various products (sheets, pipes, wire, dishes, equipment for the production of alcohols, refrigerators, gas compressors, etc.), galvanizing is often used by immersing the products in molten zinc.
4.Conclusion
In this work, I examined the concepts of thermal and chemical-thermal processing of alloys
Heat treatment is used to change the mechanical properties and structure of metals and alloys. The main methods of heat treatment are annealing, hardening and tempering. The choice of one or another heat treatment method depends on the composition of the alloy and the properties that we want to obtain, based on the analysis of phase diagrams. It is also necessary to take into account the dynamics of changes in the structure of materials.
Chemical-thermal treatment includes such types of processing of alloys as: carburizing, nitriding, nitrocarburizing, cyanidation, boriding, siliconizing, diffusion metallization of steel, etc.
One of the most effective and universal chemical-thermal treatment processes is boriding.
Boriding is used to increase the wear resistance of the surface layer of a steel product, in particular at elevated temperatures, to increase its hardness and wear resistance.
Products that have undergone boriding have increased scale resistance up to 800 °C and heat resistance up to 900–950 °C. The hardness of the borated layer in pearlitic steels is 15,000–20,000 MPa.
Types of metal that can be processed
There are three main groups of metal used for hardening:
- Steel with non-hardening core. This group includes the following grades of steel suitable for cementing - 20, 15 and 10. These parts are small in size and are used for use in domestic conditions. During hardening, austenite is transformed into a ferrite-pearlite mixture.
- Steel with a weakly hardened core. This group includes metals of such grades as 20Х, 15Х (low-alloy chromium steels). In this case, an additional ligation procedure is performed using small doses of vanadium. This ensures a fine grain, which results in a more ductile and ductile metal.
- Steel with a highly hardened core. This type of metal is used for the manufacture of parts with complex configurations or large cross-sections that can withstand various shock loads and are exposed to alternating current. During the hardening process, nickel is introduced or, if it is deficient, manganese is used, while small doses of titanium or vanadium are added to crush the grain.
Chemical reactions[edit]
Spherical model of the complex anion aurocyanide or dicyanoaurate (I), - . [10]
"Pile" of cyanide leaching at a gold mine near Elko, Nevada
The chemical reaction for dissolving gold, the "Elsner equation", is as follows:
4 Au (s) + 8 NaCN (aq) + O 2 (g) + 2H 2 O (l) → 4 Na (aq) + 4 NaOH (aq)
In this redox process, oxygen removes through a two-step reaction one electron from each gold atom to form the Au(CN)-2ion complex. [eleven]
Stages of nitrocarburization
The nitrocarburization process includes two stages:
- carbon-nitrogen saturation of the surface layer of the metal for one and a half to two hours;
- subsequent carbon saturation of the upper layers of steel with desorption of some nitrogen atoms.
After completion of the process, the metal product has the necessary properties: bending strength, reduced sensitivity to stress, and ductility. In addition, nitro-carburized surfaces achieve corrosion resistance and high hardness.
For parts that have undergone nitrocarburization, inspection is required: routine inspection to identify visible traces of oxidation, coking, brown deposits, as well as physical defects (chips, nicks, etc.); selective control of the resulting hardness using Super-Rockwell or Vickers instruments; selective control of the fragility of the treated layer using a Rockwell device. Typically, samples made from the same material as the parts being processed are tested. These samples, together with the parts, must undergo heat treatment and nitrocarburization.
It is also necessary to monitor the composition of the gases leaving the furnace (perform a chemical analysis); check gas flow with rheometers; check the consumption of carburizers and triethanolamine.
Advantages of nitrocarburization over cementation
- The critical points of transformations shift to lower temperatures. This allows the process temperature to be reduced to 810-850°C. This temperature, compared to the carburization temperature (910-1050°C), leads to much less warping of products;
- Due to the relatively low temperatures of the process, austenite grains during nitrocarburization can grow much less than during carburization during the carburization process;
- The nitrocarburization process in some cases is much faster than the cementation process. In this case, most often, there is no need to harden with repeated heating, as with carburizing.
Properties of metal after processing
After carburization, the strength of the workpieces used increases. A carburized layer is formed on the surface of the metal. Its hardness on alloy steels does not exceed 58–61 HRC, and in metals with low carbon content – 60–64 HRC. To remove large grains formed after heat treatment, the workpiece is heated again and then tempered.
Additional hardening to correct defects should be carried out at a temperature of 900 degrees. Large grains are crushed due to the formation of perlite and ferrite. When it comes to alloy steel, normalization is carried out as an additional treatment. At the final stage of processing, the workpiece is subjected to low-temperature tempering.
Steel release (Photo: Instagram / rosneftegazinstrument)
The purpose of steel cyanidation and the essence of technology
The primary goal of cyanidation is to strengthen the surface layer of steel of various parts, giving it a higher endurance limit, since this layer is subject to the greatest loads during operation of mechanisms and structures. Saturation of the surface layer of the metal with carbon and nitrogen is usually used due to their rapid penetration when they interact simultaneously. The following types of metal can be processed using cyanidation:
- any stainless steel;
- alloyed steel alloys or those without the presence of alloying components, steels with an average carbon concentration;
- steel for structural purposes, where there is little carbon present.
The chemical-thermal cyanidation method adheres to the following technology:
- The part is placed in a working bath with molten cyanide salt of the composition 15% Na₂CO₃, 60% NaCl and 25% NaCN.
- Next, the working medium is heated to a temperature from 930 to 530 degrees Celsius (depending on the selected processing mode).
- Carbon monoxide and nitrogen released from the salt saturate the metal for several hours.
The essence of the process by which carbon and nitrogen can penetrate into a layer of steel is diffusion. During the technology stages listed above, the process goes through two main stages, separated by time periods:
- The initial period of nitrocarburization lasting from one to three hours, characterized by the introduction of nitrogen and carbon atoms into the crystal lattice of the metal.
- The final period when nitrogen atoms that have previously penetrated and saturated the steel begin to desorb (leave the surface, again acquiring the state of a gas), while carbon continues to saturate the metal until the effect of temperature and working environment ends.
Statement
The ore is crushed using grinding equipment. Depending on the ore, it is sometimes further concentrated using froth flotation or. Water is added to make a paste
or
pulp
. The base ore slurry may be combined with a solution of sodium cyanide or potassium cyanide; many operations use calcium cyanide, which is more economical.
To prevent the creation of toxic hydrogen cyanide during processing, slaked lime (calcium hydroxide) or soda (sodium hydroxide) is added to the extractant solution to ensure that the acidity during cyanidation is maintained over a pH of 10.5 – highly alkaline. Lead nitrate can improve gold leaching rates and quantities, especially when processing partially oxidized ores.
Effect of dissolved oxygen
Oxygen is one of the reactants consumed during cyanidation by accepting electrons from the gold, and a lack of dissolved oxygen reduces the rate of leaching. Air or pure oxygen gas can be blown through the pulp to maximize dissolved oxygen concentration. Internal oxygen-pulp contactors are used to increase the partial pressure of oxygen contacting the solution, thus raising the dissolved oxygen concentration well above the saturation level at atmospheric pressure. Oxygen can also be added by dosing the pulp with a solution of hydrogen peroxide.
Pre-aeration and ore washing
In some ores, especially partially sulfided ones, aerating (before introducing cyanide) the ore into water at high pH can make elements such as iron and sulfur less reactive to cyanide, making the gold cyanidation process more efficient. In particular, the oxidation of iron to iron(III) oxide and subsequent precipitation as iron hydroxide minimizes the loss of cyanide due to the formation of ferrous cyanide complexes. Oxidation of sulfur compounds to sulfate ions avoids the conversion of cyanide to the byproduct thiocyanate (SCN – ).