Martensite and martensitic transformation in steels
Martensite is a supersaturated solid solution of carbon in α-iron (α-Fe). Read what austenite, cementite, ferrite and pearlite are here. When eutectoid steel (0.8% carbon) is heated above point A1, the original pearlite structure will transform into austenite. In this case, all the carbon present in the steel will dissolve in austenite, i.e. 0.8%. Rapid cooling at a supercritical rate (see figure below), for example in water (600 °C/sec), prevents the diffusion of carbon from austenite, but the fcc crystal lattice of austenite will rearrange into the tetragonal lattice of martensite. This process is called martensitic transformation. It is characterized by the shear nature of the restructuring of the crystal lattice at a cooling rate at which diffusion processes become impossible. The product of martensitic transformation is martensite with a distorted tetragonal lattice. The degree of tetragonality depends on the carbon content in the steel: the more it is, the greater the degree of tetragonality. Martensite is a hard and brittle structure of steel. Found in the form of plates, under a microscope they look like needles.
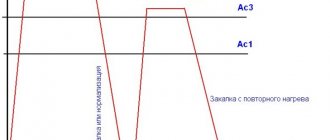
The hardening temperature for most steels is determined by the position of the critical points A1 and A3. In practice, the hardening temperature of steels is determined using steel graders. How to choose the hardening temperature of steel, taking into account points Ac1 and Ac3, read the link.
Vacation process
All hardened parts are subject to tempering. This is done to relieve internal stress. As a result of tempering, the hardness of the steel is slightly reduced and the ductility of the steel is increased.
Depending on the required temperature, tempering is carried out:
- in oil baths;
- in saltpeter baths;
- in furnaces with forced air circulation;
- in baths with molten alkali.
The tempering temperature depends on the grade of steel and the required hardness of the product, for example, a tool that requires a hardness of HRC 59 - 60 should be tempered at a temperature of 150 - 200 degrees. In this case, internal stresses decrease and hardness decreases slightly.
High-speed steel is tempered at a temperature of 540 - 580 degrees. This tempering is called secondary hardening, since as a result the hardness of the product increases.
Products can be tarnished by heating them on electric stoves, in ovens, even in hot sand. The oxide film that appears as a result of heating acquires different tarnish colors, depending on the temperature. Before you start tempering one of the tarnish colors, you need to clean the surface of the product from scale, oil deposits, etc.
Usually, after tempering, the metal is cooled in air. But chromium-nickel steels should be cooled in water or oil, since slow cooling of these grades leads to temper brittleness.
Microstructure of steel after hardening
Most steels after hardening are characterized by the structure of martensite and retained austenite, the amount of the latter depending on the carbon content and the qualitative and quantitative content of alloying elements. For structural steels of medium alloying, the amount of retained austenite can be in the range of 3-5%. In tool steels this amount can reach 20-30%.
In general, the structure of steel after hardening is determined by the final requirements for the mechanical properties of the product. Along with martensite, after quenching, ferrite or cementite may be present in the structure (in case of incomplete quenching). When steel is isothermally hardened, its structure may consist of bainite. The structure, final properties and hardening methods of steel are discussed below.
Partial hardening of steel
Partial quenching is called quenching, in which the cooling rate is not sufficient for the formation of martensite and it turns out to be below critical. This cooling rate is indicated by the blue line in the figure. During partial hardening, the “nose” of the C-curve steel seems to be touched. In this case, in the structure of the steel, along with martensite, troostite will be present in the form of black island inclusions.
The microstructure of partially hardened steel looks something like this:
Partial hardening is a defect that is eliminated by complete recrystallization of the steel, for example, during normalization or during reheating for hardening.
Features of the steel hardening process
Regardless of which steel hardening technology is chosen, it will consist of the following steps:
- Heating _ How long the product will be in the furnace chamber depends on the type of metal and the desired effect.
- Excerpts . Temperature and period depend on the volume of product and its characteristics. The through-heating stage allows the transformation of the steel structure to be completed.
- Cooling . Not only is the cooling medium important, but also the speed at which the process will be performed.
Chamber furnaces are best suited for processing carbon steel. It is worth considering that in this case, preheating of the sample is not required. These brands are not subject to warping or cracking of the base.
Steel hardening is a heat treatment technology that makes it easy for even inexpensive types of metal to improve their performance characteristics. As a result, it is possible to reduce the cost of production, increasing the profitability of production
The change in metal properties depends on compliance with each hardening criterion. The most significant is the heating temperature. It is this that influences the change in the atomic lattice. Which temperature mark to choose and determine the holding period? The required heat treatment conditions for steel depend on the required level of strength and hardness for the longest possible service life of the product, with increased wear.
Chamber furnaces for heat treatment of different grades of steel are made with different sizes of working chambers and methods of loading samples. You can choose the appropriate option based on production volumes
Incomplete hardening of steels
Quenching at temperatures lying between A1 and A3 (incomplete quenching) retains in the structure of hypoeutectoid steels, along with martensite, part of the ferrite, which reduces the hardness in the quenched state and worsens the mechanical properties after tempering. This is understandable, since the hardness of ferrite is 80HRC, and the hardness of martensite depends on the carbon content and can be more than 60HRC. Therefore, these steels are usually heated to temperatures 30–50 °C above A3 (full hardening). In theory, incomplete hardening of steels is not permissible and is considered a defect. In practice, in some cases, incomplete quenching can be used to avoid quenching cracks. Very often this concerns hardening with high frequency currents. With such hardening, it is necessary to take into account its feasibility: type of production, annual program, type of product responsibility, economic justification. For hypereutectoid steels, quenching at temperatures above A1 but below Acm produces excess cementite in the structure, which increases the hardness and wear resistance of the steel. Heating above the temperature Acm leads to a decrease in hardness due to the dissolution of excess cementite and an increase in retained austenite. In this case, the austenite grain grows, which also negatively affects the mechanical characteristics of the steel.
Thus, the optimal quenching for hypoeutectoid steels is quenching from a temperature 30–50 °C above A3, and for hypereutectoid steels – at 30–50 °C above A1.
The cooling rate also affects the hardening result. The optimal cooling medium is one that quickly cools the part in the temperature range of minimum stability of supercooled austenite (in the range of the nose of the c-curve) and slowly in the temperature range of martensitic transformation.
Cooling stages during hardening
The most common quenching media are water of various temperatures, polymer solutions, alcohol solutions, oil, molten salts. When hardening in these environments, several cooling stages are distinguished:
— film cooling, when a “steam jacket” is formed on the surface of the steel;
- nucleate boiling, which occurs with the complete destruction of this steam jacket;
— convective heat transfer.
More details about the cooling stages during quenching can be found in the article “Characteristics of quenching oils”
In addition to liquid quenching media, cooling in a gas flow of different pressures is used. It can be nitrogen (N2), helium (He) and even air. Such quenching media are often used in vacuum heat treatment. Here it is necessary to take into account the fact of the possibility of obtaining a martensitic structure - the hardenability of steel in a certain environment, i.e. the chemical composition of the steel on which the position of the c-curve depends.
Hardening
Steel hardening is a heat treatment process, the essence of which is to heat steel to a temperature above the critical temperature, followed by rapid cooling. As a result of this operation, the hardness and strength of steel increase, and ductility decreases.
When steels are heated and cooled, the atomic lattice is rearranged. The critical temperature values for different grades of steel are not the same: they depend on the content of carbon and alloying impurities, as well as on the rate of heating and cooling.
After hardening, the steel becomes brittle and hard. When heated in thermal furnaces, the surface layer of products becomes covered with scale and is decarbonized the more, the higher the heating temperature and the holding time in the furnace. If the parts have a small allowance for further processing, then this defect is irreparable. Hardening modes for hardening steel depend on its composition and technical requirements for the product.
During hardening, parts should be cooled quickly so that austenite does not have time to transform into intermediate structures (sorbitol or troostite). The required cooling rate is ensured by selecting the cooling medium. In this case, excessively rapid cooling leads to cracks or warping of the product. To avoid this, in the temperature range from 300 to 200 degrees, the cooling rate must be slowed down, using combined hardening methods. The method of immersing the part in a cooling medium is of great importance to reduce warping of the product.
Factors influencing the position of c-curves:
- Carbon. Increasing the carbon content to 0.8% increases the stability of supercooled austenite, and accordingly the c-curve shifts to the right. When the carbon content increases above 0.8%, the c-curve shifts to the left;
— Alloying elements. All alloying elements increase the stability of austenite to varying degrees. This does not apply to cobalt; it reduces the stability of supercooled austenite;
— Grain size and homogeneity. The larger the grain and the more uniform its structure, the higher the stability of austenite;
— An increase in the degree of distortion of the crystal lattice reduces the stability of supercooled austenite.
Temperature affects the position of c-curves through all of the above factors.
Methods of hardening steels
In practice, various cooling methods are used depending on the size of the parts, their chemical composition and the required structure (diagram below).
Diagram: Cooling rates for different methods of hardening steels
Continuous hardening of steel
Continuous hardening (1) is a method of cooling parts in one environment. After heating, the part is placed in a quenching medium and left there until completely cooled. This technology is the most common and is widely used in mass production. Suitable for almost all types of structural steels.
Hardening in two environments
Quenching in two environments (speed 2 in the figure) is carried out in different quenching environments, with different temperatures. First, the part is cooled in the temperature range, for example, 890–400 °C, for example in water, and then transferred to another cooling medium - oil. In this case, the martensitic transformation will already occur in an oil environment, which will lead to a decrease in the leash and warping of steel. This hardening method is used for heat treatment of stamping tools. In practice, the opposite technological technique is often used - first the parts are cooled in oil and then in water. In this case, the martensitic transformation occurs in oil, and the parts are moved into water for faster cooling. This saves time on implementing the hardening technology.
Step hardening
During stepwise quenching (speed 3), the product is cooled in a quenching medium having a temperature higher than the martensitic transformation temperature. In this way, a certain isothermal holding is obtained before the transformation of austenite into martensite begins. This ensures uniform temperature distribution over the entire cross-section of the part. This is followed by final cooling, during which the martensitic transformation occurs. This method produces hardening with minimal internal stress. Isothermal holding can be done just below the temperature Mn, after the start of the martensitic transformation (speed 6). This method is more difficult from a technological point of view.
Isothermal hardening of steels
Isothermal hardening (speed 4) is done to obtain the bainitic structure of the steel. This structure is characterized by an excellent combination of strength and plastic properties. During isothermal hardening, parts are cooled in a bath of molten salts, which have a temperature 50–150 °C above the martensite point Mn, maintained at this temperature until the end of the transformation of austenite into bainite, and then cooled in air.
When hardening onto bainite, it is possible to obtain two different structures: upper and lower bainite. Upper bainite has a feathery structure. It is formed in the range of 500-350°C and consists of lath-shaped ferrite particles <1 µm thick and 5-10 µm wide, as well as thin cementite particles. The structure of upper bainite is characterized by higher hardness and strength, but lower ductility. Lower bainite has a needle-like martensite-like structure and is formed in the range of 350-200 °C. Lower bainite consists of fine particles of ε-carbides located in ferrite platelets. The bainite transformation never goes to completion. The structure always contains martensite and retained austenite. More preferable, in terms of performance characteristics, is the lower bainite structure. Products with such a structure are used in car construction, where parts experience shock-tensile stresses. The bainite hardening technology requires special hardening equipment. Additional materials on this technology can be found in the article “Technology of hardening for bainite.”
Cold treatment (5) is used for steels in which the temperature of the end of the martensitic transformation Mk is below room temperature.
High-speed steels, cemented parts, measuring instruments, and other particularly precise products are subjected to cold treatment. You can read more about this non-standard method of heat treatment in the article “Cold processing of steel parts”
Hardening technology for different grades of steel - how and why it is done
According to GOST for heat treatment of steel, hardening of different grades can be:
- With one cooler . The sample, brought to a certain temperature, is immersed in liquid. The metal remains there until it cools to the required level. The method is used for carbon and alloy materials, as well as products with a simple design.
- Intermittent . Two environments are used. The metal first undergoes rapid cooling. Water is suitable for this. The products are then immersed in oil. This is necessary to slowly reach a certain temperature mark. The method is used for high-carbon steel.
With different hardening methods, not only the resulting quality characteristics of the steel may differ, but also the colors of the heat.
- Stepped . Products are cooled in an environment whose thermal temperature exceeds the martensitic level of the grade being processed. During cooling and holding, the part along its entire perimeter reaches the temperature of the quenching container. After this, slow cooling with quenching is carried out. This is how austenite transforms into martensite.
- Jet . The surface is intensively sprayed with water pressure. In this case, a steam cocoon is not formed, thanks to which deep calcination can be achieved. Used if it is necessary to treat only part of the surface.
- Isothermal . The method is similar to step hardening, but differs in the exposure time. The steel remains in the environment exactly as long as necessary to complete the isothermal transformation of austenite.
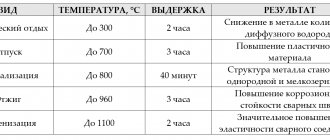
Basic temperature and time conditions for heat treatment of steels - table of indicators for different grades
Dependence of martensite hardness on carbon content
The hardness of steel after quenching depends on the hardness of martensite, which in turn depends on the carbon content. As the carbon content increases, the hardness after hardening of the steel also increases. The graphical dependence is shown in the figure.
Graph of martensite hardness versus carbon content
Solvent for alkyd enamel - https://www.dcpt.ru