Manganese steels
Manganese as an alloying element is widely used in powder metallurgy. Just like nickel, it belongs to the transition metals. Manganese expands the range of existence of y-Fe, significantly increases the hardness of ferrite, increases the stability of supercooled austenite and reduces the temperature of martensitic transformation. Manganese significantly increases the hardenability of powder steels. It is a carbide-forming element. With carbon it forms Mn3C carbide, which is more stable and durable than iron carbide (cementite). When manganese is introduced into iron-carbon alloys, pure manganese carbides are not formed, but complex (double) carbides of the cementite type (Fe, Mn)3C are always obtained, in which some of the iron atoms are replaced by manganese atoms. Its content in cementite is determined by its amount in steel. In high-manganese steel of the austenitic class, such a double carbide contains more manganese than iron (about 80% Mn and 20% Fe), and in medium-manganese steel with a content of less than 3% Mn, on the contrary, such a carbide contains more iron than manganese (about 80 % Fe and 20% Mn).
The late 70s and early 80s were characterized by growing interest in powdered manganese steels, driven by the need to develop inexpensive alloyed powder steels for mass production. However, the use of manganese (as well as chromium) as an alloying element to produce powder steels is associated with a number of difficulties due to the high affinity of these elements for oxygen.
To reduce the degree of oxidation of manganese and the formation of difficult-to-reduce oxides during the sintering process, it is recommended to use pure starting components and dried sintering media. In addition, it is proposed to introduce HCl, HBr, HF into the sintering medium or introduce boric acid or metal borates into the charge, and use getter fills containing ferroaluminum or ferrosilicon. Manganese can be added to iron powder in the form of crushed ferromanganese or a special alloy. On the contrary, the authors of the work, studying the process of producing manganese steels from a mixture of powders, come to the conclusion that the decisive process should be considered the sublimation of manganese and the formation of a gas phase during sintering. Manganese vapor, settling on iron particles, activates the diffusion of the alloying element. For the most effective effect of sublimation on the alloying and sintering process, in the opinion of the author, manganese should be added in the highest concentration. Under such conditions, manganese vapor released from the compact interacts with oxygen in the protective environment, and the resulting oxides are carried away by the flow and are not formed in the volume of the material.
A number of authors note a decrease in the amount of manganese in the workpiece during the sintering process due to its evaporation. In this case, the loss of the alloying component depends on the proportion of open porosity. An increase in pressing pressure helps to suppress the process of evaporation and entrainment of manganese.
The processes of sintering and structure formation of manganese steels were studied in the work. Powders of reduced and electrolytic iron, ferromanganese with 78% manganese, and graphite were used as starting materials. Sintering was carried out in vacuum at a temperature of 1100 °C. Shrinkage of steels and mechanical properties after sintering are given in table. 31.
The decrease in shrinkage with increasing manganese content is obviously associated with an increase in porosity. It has been established that pores are located in the centers of extended regions of austenite in manganese formed by fine-plate pearlite. It is noted that the strength of sintered steels in all cases was noticeably lower than the strength of cast and heat-treated steels, which, according to the author, is a consequence of the heterogeneity of the material. This leads to the need to introduce a higher amount of alloying elements than should be based on traditional practice.
The structure formation and properties of manganese steels based on atomized and reduced iron powders were studied in the work. Ferromanganese carbon (75% Mn; 7.7% C) with a particle size of 0.04 mm was used as an alloying additive.
The type of iron powder has a significant influence on the structure and properties of sintered steels. When using atomized powder during sintering, the boundaries of the iron powder particles are preserved, and the core of the particles remains ferritic, unalloyed. On the contrary, when using reduced powder, no boundaries of the original particles are observed in the microstructure. It has been established that the increasing strength depends almost linearly on the manganese content up to a content of 4-4.5%, at which a maximum is observed. The strength of samples based on reduced powder increases by 210 MPa for each percent of the alloying element, and for samples based on atomized powder with the same amount of carbon, the increase in strength is 108 MPa for each percent of manganese. The maximum strength was achieved on steels based on reduced iron powder with 4.2% manganese and 0.2% carbon and amounted to 886 MPa, while the maximum strength value of steels based on atomized powder was 672 MPa. Steels based on reduced powder have higher elongation values and lower hardness than steels based on atomized powder.
The work investigated the influence of technological parameters and composition on the properties of sintered alloys and steels: Fe-Mn, Fe-Mn-C, Fe-Mn-Cr, Fe-Mn-Cr-C, Fe-Mn-Cr-Mo-C. The compositions were obtained by mechanical mixing of iron powder and alloying elements introduced in pure form or in the form of a ferroalloy. Iron powder obtained by atomization (atomet), electrolytic manganese powder (particle size
Antiferromagnetic steels with manganese
Austenitic antiferromagnetic steels with special physical properties have been developed and are being used. The main alloying element in steels of this group is manganese, the content of which should provide an austenitic structure (
20 % ). To impart high strength, steel is alloyed with tungsten and vanadium (50G20FV7, 50G20Kh4FV7, etc.). Tungsten has a low linear expansion coefficient, which promotes the formation of manganese austenite with low thermal expansion coefficient values.
A large group of manganese-containing complex alloy steels is also known, in which hardening is achieved due to the formation of excess precipitation phases (carbides, nitrides, intermetallic compounds, elements V, W, Mo, Nb, Ti, Ta, Zr, Al). These steels are widely used for the production of pipes (45G17Yu3, 45G15, N9Kh3F2Yu, etc.).
Manganese steel - grade
Low-carbon manganese steels of grades 10G2A and 12G2A have high ductility and good weldability. They are used for the manufacture of stamp-welded parts. [1]
When processing manganese steel grade G12 (manganese content 12–94%), it was found that the most suitable for such processing is a cutter made of hard alloy grade T15K6, which has sufficient durability at a cutting speed of 13–6 m/min. The T5KYu alloy provides satisfactory durability (50 min. However, the T15K6 alloy is relatively brittle and does not work well under impact loads. [2]
Crankshaft 3 is made of manganese steel grade 50G and rests on five main bearings. The surfaces of the shaft journals are hardened by high frequency currents. The diameter of the main neck is 88-9 mm, the diameter of the crank is 70 mm. To balance the centrifugal forces, counterweights are installed on the first and fourth cranks of the shaft. [3]
Steel with high wear resistance is manganese steel grade G13, containing 1 0 - 1 3% C; 11 0 - 14 0% MP. It belongs to the austenitic class. [4]
For welding main pipelines, wire made of carbon steel grades SV-08 and SV-08A and manganese steel grades SV-08g-A is used. The letter A in the wire grade means that the wire contains significantly less harmful impurities - sulfur and phosphorus, therefore such wire is used for more demanding work. [5]
The regenerator consists of housing 1, the lower part of which (up to the flange connection) is made of chromium-nickel steel grade X18N9T, and the upper part is made of manganese steel grade 09G2DT / m; 2 coils made of copper or steel tubes, and 3 stone nozzles with a granule size of 4 - 10 mm. The coils rest on the bottom of the housing. The coil collectors are routed through seals 4 and 5, located in the bottom and cover. Air is introduced into the regenerator and the return flow is output through a perforated cone 6, covered with a stainless steel mesh 7, and the air is removed and the return flow is introduced through an annular perforated manifold 8, also covered with a stainless steel mesh. [6]
Alloy steels and iron-based alloys with special properties contain a large number of alloying components, the combination of which gives the steels heat resistance, anti-corrosion, high electrical resistance and other valuable properties. For example, steel grade 1Х18Н9Т - chromium-nickel stainless steel containing about 0.1% carbon, 18% chromium, 9% nickel, about 1% titanium, is highly acid-resistant and is used for the manufacture of devices in chemical engineering factories; manganese steel grade PZ, called Hadfield steel, containing from 11 to 14% manganese, works well against abrasion and is used for the manufacture of bucket teeth for excavators and railway switches. [7]
Alloy steels and iron-based alloys with special properties contain a large number of alloying components, the combination of which gives the steels heat resistance, anti-corrosion, high electrical resistance and other valuable properties. For example, steel grade 1Х18Н9Т - chromium-nickel stainless steel containing about 0 1% carbon, 18% chromium, 9% nickel, about 1% titanium, is highly acid-resistant and is used for the manufacture of devices in chemical engineering factories; manganese steel grade G13, called Hadfield steel, containing from 11 to 14% manganese, works well against abrasion and is used for the manufacture of bucket teeth for excavators and railway switches. [8]
In bins designed to store solid lump materials, the inner surface of the inclined walls is lined to protect the walls from abrasion and dents due to impact. The type of lining depends on the abrasive properties of the bulk material. Thus, bunkers for ore and scrap are lined with sheet manganese steel grade ZOG2 with a thickness of 6 - 10 mm. Sometimes wooden lining is used. [10]
Armor for crushers - choosing the optimal alloy
An important component of the cost of operating a crushing and screening plant is the cost of wear elements. And the first thing that comes to mind when you hear wear elements is armor, plates for crushers. This note will talk about ways to optimize this expense item or how to make wearable items last longer.
First, briefly what the impact is:
- compression
- shift
- hit
- glancing impact or abrasion
In our country, the most common alloy used for the production of armor is manganese steel. But potassium permanganate is not always the best solution. How to choose which alloy is optimal for the task? To do this, it is necessary to study the physical properties of alloys.
Manganese engineering steel of pearlitic class
- Perlite grade manganese mechanical steel 1.0-2.0% MP per-eutectoid machine during the production of manganese steel is easy to process and has deep quenching property. Due to its mechanical properties, in comparison with carbon steel, such steel in products with a diameter of 30-40 mm has fairly good toughness and high strength. At 0.1-0.7% C (up to 0.05-0.10%), manganese steel is used in industry in machine production at approximately 0.7-1.8% of the 20 MP grade. In the table.
Figure 19 shows the chemical composition and critical points of Jurassic-Mars-Pearl rock steel of some of the most common grades. Table 19 average manganese steel Steel grade 15g 20g ZOG 40g 50g 60g 70g 30G2 40G2 50G2 C% 0.12-0.18 0.17-0.24 0.27-0.34 0.37-0.44 0.47-0.55 0.57-0.65 0.67 -0.75 0.27-0.34 0.37-0.44 0.47-0.55 MP 、% 0.7-1.0 0.7-1.0 0.7-1.0 0.7-1.0 0.7-1.0 0.7-1.0 0.9-1.2 1.4-1.8 1.4-1.8 1.4-1.8 Critical point°C How( 720. 720. 720. 720. 720. 72 0. 720. Seven hundred ten Seven hundred ten Seven hundred ten As. 880. 850. Eight hundred ten Seven hundred ninety Seven hundred seventy Seven hundred sixty 740. Seven hundred ninety Seven hundred seventy Seven hundred fifty Note 1 silicon content 0.17
0.37%, sulfur and phosphorus in amounts of 0.08% or less. 15 g and 20 g steel have increased strength, high toughness, and are susceptible to
cold plastic deformation and mechanical processing, weld well. Lyudmila Firmal
Such steels are often used in various welded structures without heat treatment and after normalization from 900-940°, such as bolts, nuts, rivets, etc. Manganese steel, including 0.15〜0.20% C, is also used as cement for products without heavy loads. When cementing this steel, there is a smooth transition from the cement layer to the non-cement core of 96 MN steel. After cementing the air-body, the surface hardness of the product Ain is uniform (no soft spot).
Cementation of manganese steel is carried out at 900-920°, and the duration of carburization to obtain a layer of a certain depth is approximately the same as that of ordinary carbon steel. To eliminate overheating and grinding of grain products 160. 120. South Ossetia $.Eighty * o » az Forty Go. В • * ^ — Eight ^ L H y y N. N. N. Eighty 70. 60. Fifty 」 Thirty Twenty / la Three hundred billion four hundred million five hundred thousand six hundred Temperature, One hundred 30. Mechanical properties of 50G2 steel after hardening from 800° and curing at different temperatures. In the case of manganese steel, after carburization, it is cooled with oil 820-840° to make intermediate hardening, then it finally hardens and gives the cement a high hardness layer. In some cases, instead of primary (intermediate) hardening, cement products are normalized into a box with a well-developed carburetor.
- The final note is 780 to 800°. Large cured products are cooled with water or water in oil, and small cured products are cooled with oil. The holiday takes place at a temperature of 160-180°. Modified low carbon steels ZOG, 30G2, 40G and 40G2 are hardened with water or oil (depending on the size of the product) and cooled at 830-880° (depending on the critical point). Tempering is given at a given hardness at 450-650°. This steel is used in the manufacture of products such as warm, semi-axial and lever.
Steel with a medium carbon content (50G and 50G2) is oil-hardened at 820-840°, and after tempering at 450-600° this steel acquires high strength with a sufficient pearlite content of grade 97 manganese structural steel. 2.0 Toughness. This steel is used in similar products as crankshafts, connecting rods and axles. Steel with a high carbon content (60 g and 70 g) is hardened at 800-820°C with oil cooling, and the tempering temperature of a given hardness is 200-450°.
Such steels are used for cold impact stamping of tools (crimps, dies, sledgehammers, etc.), as well as springs, springs, friction discs, etc. Lyudmila Firmal
In Fig. Figure 30 shows how the tempering temperature affects the change in the mechanical properties of 50G2 steel after oil quenching from 800°C. Under annealing conditions, the tensile strength of this steel is about 70 kg
Austenite structure
The austenitic structure is distinguished by the polyhedral shape of the grains, within which characteristic twins are often observed, shown in Figure 1. During oxidative etching of a polished section, a thin oxide film appears on it, which has a different thickness on the surface of each grain, depending on the crystallographic orientation of the grain. Thus, instead of a smooth surface of the polished section, a characteristic relief in the form of depressions and protrusions is formed on it. Figure 2, 3, 4, 5 schematically shows austenite grains. Very often, in the structure of manganese austenitic steels, thin lines appear covering the austenite grains. These lines appear due to the occurrence of internal stresses during cold deformation, as well as during solidification of the metal, and in some cases during hardening. These fine lines very often do not disappear even after subsequent heat treatments. The structure of such austenitic steel is shown schematically in Figure 6. In the structure of the metal of castings, a typical dendritic structure can often be observed (Figure 7). A highly developed and pronounced network of dendritic structure.
Welding manganese steels
Author:
Igor
Date of:
28.12.2018
- Article
- Photo
- Video
Manganese structural steel for special purposes has a unique combination of strength and toughness, which is used for the manufacture of armor, tracks, tanks, leaf springs, springs. The products are characterized by high wear resistance to abrasion and shock loads. They are produced by casting, but during operation they often require welding of manganese steels. This can be either the creation of a new design or the fusing of a worn part.
The weldability indicator is the carbon equivalent, the formula of which includes: C, Mn, Si, Cr, Ni, Cu - arrangement according to influence. The main alloying elements are carbon and manganese: the higher their content, the more complicated the process. An alloy with C up to 0.25% is considered well weldable, but as the indicators increase, this ability decreases.
Cavitation manganese-containing steels
The main requirement for steels for this purpose is high resistance of products to intense cavitation effects, i.e., a common type of external surface impact on elements of machines and equipment. It has been established that relaxation of local stresses as a result of pulsed, hydrodynamic effects at the interface between the medium and the surface of products made from metastable steels is best achieved if martensite is present in the steel structure. Abroad, chromium and chromium-nickel steels with additions of manganese and copper (1Х17Н6Г8, USA), the structure of which is represented by chromium-nickel and chromium-nickel-manganese austenite, are used as cavitation-resistant materials. (However, it has been established that manganese austenite, due to its metallophysical nature (lower values of stacking faults, higher degree of microdistortion), is characterized by lower dislocation mobility. In this regard, a number of steel grades have been proposed containing, along with chromium (10-14%), from 10 to 12% Mn .
Features of the chemical composition of manganese steels
Important! During operation, it is necessary to ensure rapid cooling of the weld, since prolonged heating causes the release of carbides and a decrease in strength.
The presence of C 0.6-1.2%, Mn 1-14% can also be alloyed with other elements in amounts up to 1%. When melted, the bulk of the components combine with oxygen, releasing slag, carbon forms CO gas, i.e. burns out. The slag, in turn, interferes with the process: it closes the electric arc, partially enters the melt and reduces the strength of the connection. The oxidation process reduces the content of materials in the melt, which completely changes the original chemical composition, and therefore the properties.
Influence of smelting method on gas content and mechanical properties
Welding manganese austenitic steels is also complicated by structural changes in the heat-affected zone. Heating to recrystallization temperatures leads to the precipitation of carbides, grain growth, i.e., a local change in the properties of the metal due to transformation of the structure - a decrease in strength and toughness, and an increase in fragility.
Types and technologies of the welding process
The technology for welding manganese steels, regardless of the method used, must take into account all negative factors and ensure:
- Protection against oxidation. This function is partially performed by slag, what happens after its formation and why some of the elements are spent. To completely prevent the oxidation process, a protective atmosphere must be used. As a rule, this is the use of vacuum - a technology that is expensive and difficult to implement. Argon-arc welding is much more practical. It will be appropriate both in industrial conditions and private use.
- Partial or complete restoration of the chemical composition. The content of elements in the weld changes radically; in order to partially or completely replenish it, electrodes coated with similar elements are used. There are manganese and aluminum varieties with a dosed content of elements.
- Form of surfacing. When alloys burn out, they form a large amount of carbon monoxide, which not only hinders visibility. Being retained in the melt, they reduce the strength of the structure. To ensure their exit, surfacing with electrodes is carried out with widened stitches.
- Fast cooling. Prolonged heating and slow cooling of Mn steels lead to the precipitation of carbides, which reduce the strength and make the weld brittle. The optimal ratio in terms of heating and cooling rates is the electric arc method.
Welding 65G steel is difficult due to the C content. For these grades, a number of conditions are applied that reduce the consequences of intervention in the structure. Essentially, the process is the surfacing of an intermediate layer between surfaces. For this purpose, electrodes of a certain composition are used, they are selected depending on the degree of alloying.
Using electrodes containing Mn, surfacing is carried out on ordinary structural steel, thereby giving it the wear resistance inherent in Mn steels. The procedure is carried out in 4 layers, in each of which the manganese content increases.
Welding of 16GS steel is performed using the electroslag method in a protective gas atmosphere under submerged arc. It is not prone to temper brittleness and is characterized by high resistance to overheating in the heat-affected zone. Electrodes E42, E50A are recommended for surfacing.
The methods and side effects of welding 09G2S steel are similar to those described above. For the semi- and automatic method, electrode wire SV08GA, SV-YUGA, SV10G2 + flux AN-348A, OSTS-45 are used.
Welding steel 30KhGSA. Alloying with chromium and silicon in the heat-affected structure provides not only a ferrite-pearlite composition (a certain amount of bainite and martensite is formed), but also long-term cooling, which contributes to the precipitation of carbides along the grain boundaries and the appearance of increased fragility. Electrodes E55A, E60, E55 are used here.
Welding spring steel, as well as welding spring steel, is practically impossible. Grade 50HGA is not intended for welded structures. It obtains the effect of a spring during plastic deformation in a cold state, and during weldability in the heat-affected zone, the result is partial tempering and loss of strength. A compromise is the use of OK 68/82 electrodes, which are optimal for surfacing transition layers.
Welding 09G2S steel, the technology of which provides for joining in any configuration, including welding of strip steel, differs from high-alloy steel - in this case, the principle of fusion is characteristically similar to deposition. Docking can be carried out in different ways: continuous reflow with or without heating. Gaps when welding metal are allowed depending on the cross-section and type of melting - from 0.5 to 8 mm.
Features of surfacing manganese steels
Conclusion
Carbon is the basis that indicates weldability, the second most important element is manganese (content up to 1.5% has little effect on the process). If C is more than 0.25%, the possibility of performing the operation depends on additional elements. When it increases above 0.29%, it is possible to combine under special conditions using conventional electroslag remelting. When C increases by more than 0.4%, the connection is practically impossible; a special surfacing method becomes relevant. electrodes.
Domestic low-alloy steels of increased and high strength
The microstructure of manganese steels is mainly composed of austenite;
in addition, depending on the chemical composition, it may also contain martensite, pearlite, troostite and carbides. The variety of structural components, the presence of which is largely due to the heat treatment of steel, is so great that it is impossible to describe all possible structural changes within one article. Below we will consider only those basic structural components that, in the structural transformations of the metal of industrial melts, determine its properties. The figure schematically shows the structure of the various structural components. Diagrams of the main structures of manganese steels
Manganese steel grades
- LGM
- Casting
- Laboratory
- Stumping
- Heat treatment
- Mechanical restoration
- Video
- Products Steel castings Drawings (Steel)
- Drawings (Manganese steel)
- Manganese steel
- Heat resistant steel
- Casting steel
- Structural alloy steel
- Alloy steel
- Carbon steel
- Cast Iron Drawings (Cast Iron)
- Cast iron
- Chrome cast iron
- Pig iron
- Tubing
- Cast iron ring weights (UChK)
- Housings
- Art casting Art casting
- Park casting
- LGM
- Casting
- Laboratory
- Stumping
- Heat treatment
- Mechanical restoration
- Video
- Steel casting Drawings (Steel)
Drawings (Manganese steel)
- Cast Iron Drawings (Cast Iron)
- Cast iron
- Chrome cast iron
- Pig iron
- Tubing
- Cast iron ring weights (UChK)
- Housings
- Art casting Art casting
- Park casting
Wear-resistant (white) cast iron (high chromium alloy) for crusher armor
Impact strength up to 10 J/cm2. Hardness 560-590 HB (about 57-60 HRc).
The most common wear-resistant cast iron alloy in Russia, ICHKh28N2, contains 2.7-3% carbon, 28-30% chromium, 1.5-3.0% nickel, 0.8% manganese, 0.7-1.4% silicon. Carbon and silicon have a detrimental effect on the properties of the alloy: carbon increases brittleness, and silicon promotes the formation of graphite from carbon instead of cementite in the alloy structure. On the other hand, like all cast irons, having a high carbon content (over 2.14%) compared to steels and a significant chromium content (forming special chromium carbides in the alloy structure), it has increased hardness, which is an important criterion that restrains oblique impact and sliding wear. But it is significantly less durable against compression and direct impact, which does not allow it to be used for coarse and medium crushing by any method (compression, impact) and in places where metal (uncrushed material) can enter, since such an impact as well as mechanical processing of this alloy may lead to coloring of the product.
It is used for crushers with the impact principle of destruction with a small feed size: rotary crushers with horizontal and vertical shafts (the latter are quite widely used in Russia).
Manganese steels
Manganese is one of the elements that expand the solid solution region and reduce the temperature of the critical point of steel. Manganese refines the structure of steel during secondary crystallization, due to which its plastic properties increase, has little effect on the plastic properties of steel during deformation, reduces the temperature of phase transformations and reduces the rate of formation of carbides from austenite, which improves its hardenability. The formation of complex manganese and iron carbides has a significant impact on the mechanical properties of steel.
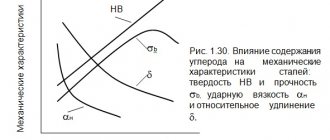
Professor of the St. Petersburg Mining Institute V.N. Lipin in 1886 began to study the effect of manganese on the properties of steel. He investigated the effect of manganese on the properties of steel at its content up to 2.5%. Three years earlier, the influence of manganese on the properties of steel with its content up to 20% began to be studied by the English researcher Hadfield. He discovered that steel containing 12% Mn had properties that were not known in other steels at that time. Steel containing 1 - 1.4% C and 10 - 14% Mn is the most common manganese austenitic steel, known as Hadfield steel. Since then, a large number of its modifications have been patented, of which a relatively small number have been mastered by industry.
Currently, cast austenitic manganese steel is widely used in industry; With its low cost and fairly simple production, this steel has high toughness, high resistance to strong impact loads, pressure and good cold hardening, due to which it has high wear resistance. Exceptionally good results are obtained when using this steel for various parts of crushers, excavators and other machines, as well as for armor plates, hammers, strikers, etc. instead of ordinary steel, which wears out quickly under these conditions.
Effect of chromium
Chromium is a metal especially often used for alloying purposes. It is added both to structural steels (for example, 20Х, 40Х) and to tool steels (9ХС, Х12М). Moreover, the final properties of chromium-alloyed steel strongly depend on its content in it. At low (less than 0.5...0.7%) concentrations, the steel structure becomes coarser and more sensitive to the direction of its subsequent processing , especially when cold rolling and bending. The uniformity of distribution of the main components of the microstructure also deteriorates.
As noted above, one of the main goals of alloying is the formation of metal carbides in steel , the strength and hardness of which is noticeably higher than that of the base metal. Chromium forms two types of carbides: hexagonal Cr7C3 and cubic Cr23C6, and in both cases the strength and cold resistance of steel increases. A special feature of chromium carbides is the presence of other elements in their structure - iron and vanadium. As a result, the temperature of effective dissolution decreases, which, in turn, leads to such positive features of chromium-alloyed steels as hardenability, the possibility of secondary dispersion hardening and heat resistance. Therefore, steels alloyed with chromium have increased operational resistance under difficult operating conditions.
However, an increase in chromium content in steel also leads to negative consequences. When its concentration is more than 5...10%, the carbide homogeneity of the material sharply deteriorates, which is accompanied by undesirable phenomena during its mechanical processing : even when heated, the ductility of steel is low, therefore, when forging with large degrees of deformation, high-chromium steels are susceptible to cracking.
With excessive carbide formation, the number of stress concentrators also increases , which negatively affects the resistance of such steels to dynamic loads. Taking this into account, the chromium content in steels should not exceed 5..6% .
Vanadium influence
Vanadium is more often used as a component of complex alloying. Its presence gives alloy steels a more uniform and favorable structure , which changes little even with heat treatment. In addition, vanadium stabilizes the γ-phase, which increases the resistance of steel to shear stress (as is known, it is during shear deformations that metals have the lowest strength).
Vanadium has virtually no effect on the hardness of steel ; this is especially noticeable for structural steels, which contain less carbon than tool steels. In complex alloy steels, vanadium increases heat resistance, which increases their resistance to brittle fracture. In this sense, the effect of vanadium is opposite to that of molybdenum. A feature of the heat treatment of alloy steels containing vanadium is the impossibility of performing high tempering after hardening, since the subsequent ductility of the steel is reduced. Therefore, in steels intended for the manufacture of large parts or forgings, the percentage of vanadium is limited to 3..4%.
Doping and impurities - is there a difference?
From a formal point of view, some chemical elements contained in ordinary steels, both structural and ordinary quality, can also be called alloying. These include, for example, copper (up to 0.2%), silicon (up to 0.37%), etc.
The reason is that any impurity is a consequence of either the purity of the original ore (manganese) or the specific metallurgical smelting processes (sulfur, phosphorus). Theoretically, steel smelted without copper, phosphorus and sulfur would have the same mechanical properties. Alloying has as its ultimate goal precisely the improvement of certain technical characteristics of steel. At the same time, phosphorus and sulfur clearly classified as harmful but inevitable impurities . The presence of copper increases ductility, but contributes to the adhesion of the surface of a metal having an excessive (more than 0.3%) concentration of copper on the surface of an adjacent part. When the structure operates under conditions of intense friction, this is a major drawback.
The presence of a chemical element with a concentration of more than 1% provides grounds for introducing its symbol into the steel grade. In addition to the aforementioned 65G steel, aluminum (present, in particular, in O8Yu steel) also receives a similar honor. In this case, aluminum is introduced into ordinary O8 structural steel in order to deoxidize it , and the fact that its ductility slightly increases is only a fortunate accompanying circumstance. Boriding steel provides it increased subsequent deformability , therefore even microadditives of boron to the chemical composition of steel are marked accordingly by changing its markings (for example, in steel 20P there is only 0.001...0.005% boron).
In general it is accepted that:
- Steels containing only one element intentionally introduced into the composition;
- Steels containing chemical elements other than carbon and manganese in an amount not exceeding 1%
- are not considered doped. On the other hand, if the percentage of iron in the melted alloy does not exceed 55%, then such material can no longer be called alloy steel.
Effect of silicon, manganese and cobalt
Silicon is the only non-metal “allowed” for alloying processes. This is explained by two factors - the low cost of the element and the clear dependence of hardness on the percentage of silicon in steel. That is why silicon is often used in the smelting of inexpensive low-alloy construction steels, as well as steels for which the optimal combination of strength and elasticity is important for operational durability. Most often, manganese is used together with silicon - examples include steel 09G2S, 10GS, 60S2, etc.
In tool steels, silicon is rarely used as an alloying component, and only in combination with other metals that neutralize its negative properties - low operational ductility and toughness. Of these steels - in particular, 9ХС, 6Х3С, etc. — produce cutting and stamping tools , which require a combination of high hardness and resistance to sudden loads.
Like silicon, cobalt, when introduced into the steel structure, does not form its own carbides, but in complex alloy steels it intensifies their formation during tempering. Therefore, cobalt is not used independently, but in combination with metals such as vanadium, chromium, tungsten , and, due to the scarcity of cobalt, its content usually does not exceed 2.5...3%.
Effect of Nickel
Nickel is the only alloying component of steel that increases its ductility and reduces hardness . Therefore, steel is not alloyed with nickel alone . But in combination with manganese, nickel leads to a noticeable increase in the hardenability of steel, which is very important in the manufacture of large machine parts for which high operational durability is important. At the same time, the presence of nickel reduces the requirements for the accuracy of compliance with the temperature ranges of heat treatment.
Alloying with nickel has a number of features. In particular, nickel, without forming its own carbides, contributes to an increase in the accumulation of “foreign” carbides along the grain boundaries, as a result of which heat resistance decreases and fragility increases in the range of 20...400 0 C. Therefore, the percentage of nickel in alloy steels is strictly linked to the presence of manganese and chromium: if they are present, the maximum concentration of nickel is 2%, and if they are absent - no more than 0.5...1%.
Alloy steels for special areas of use also contain a number of other metals (for example, titanium, aluminum, etc.). The choice of steel type is dictated by operational and financial considerations.
The influence of carbon, permanent impurities and alloying elements on the properties of steel
Steel is a multicomponent alloy containing carbon and a number of permanent or inevitable impurities Mn, Si, S, P, O, N, H, etc., which affect its properties. The presence of these impurities is explained by the difficulty of removing some of them during smelting (P, S), their transfer into steel during its deoxidation (Mn, Si) or from the charge - alloyed scrap metal (Cr, Ni, etc.). The same impurities, but in larger quantities, are also present in cast iron.
Carbon influence
. The structure of steel after slow cooling consists of two phases - ferrite and cementite. The amount of cementite increases in steel in direct proportion to the carbon content.
Cementite particles increase resistance to deformation, and in addition they reduce ductility and toughness. As a result, with an increase in carbon in steel, hardness, tensile strength, and yield strength increase, and relative elongation, relative contraction, and impact strength decrease.
Effect of silicon and manganese
. The silicon content in carbon steel as an impurity usually does not exceed 0.35–0.4%, and manganese 0.5–0.8%. Silicon and manganese are transferred into steel during its deoxidation during smelting. They deoxidize steel, i.e., combining with the oxygen of iron oxide FeO, they pass into slag in the form of oxides; deoxidation improves the properties of steel. Silicon, by degassing the metal, increases the density of the ingot.
Silicon remaining after deoxidation in solid solution (in ferrite) greatly increases the yield strength. This reduces the ability of steel to draw and especially cold heading. In this regard, in steels intended for cold stamping and cold heading, the silicon content should be reduced.
Effect of sulfur
.
Sulfur is a harmful impurity in steel. With iron it forms the chemical compound FeS, which is practically insoluble in it in the solid state, but soluble in the liquid metal. The FeS compound forms a low-melting eutectic with iron with a melting point of 988 °C. This eutectic is formed even at very low sulfur contents. Crystallizing from the liquid upon completion of solidification, the eutectic is predominantly located along the grain boundaries. When steel is heated to the rolling or forging temperature (1000–1200 °C), the eutectic melts, the bond between the metal grains is broken, as a result of which, when the steel is deformed, tears and cracks appear at the locations of the eutectic. This phenomenon is called red brittleness
.