Alloy steel is steel containing special alloying additives that can significantly change a number of its mechanical and physical properties. In this article we will understand what the classification of alloy steels is, and also consider their markings.
Alloy steel round bars
Classification of alloy steels
Based on the carbon content of steel, it is divided into:
- low-carbon steels (up to 0.25% carbon);
- medium carbon steels (up to 0.25% to 0.65% carbon);
- high-carbon steels (more than 0.65% carbon).
Depending on the total amount of alloying elements that alloy steel contains, it can belong to one of three categories:
- low alloy (no more than 2.5%);
- medium alloyed (no more than 10%);
- highly alloyed (from 10% to 50%).
The properties of alloy steels are determined by their internal structure. Therefore, the classification of alloy steels implies division into the following classes:
- hypoeutectoid - the composition contains excess ferrite;
- eutectoid - steel has a pearlite structure;
- hypereutectoid - their structure contains secondary carbides;
- ledeburite - the structure contains primary carbides.
According to their practical application, alloyed structural steels can be: structural (divided into machine-building or construction), tool, and also steels with special properties.
Purpose of structural alloy steels:
- Mechanical engineering - used for the production of parts for various mechanisms, body structures, and the like. They differ in that in the vast majority of cases they undergo heat treatment.
- Construction - most often used in the manufacture of welded metal structures and are subjected to heat treatment in rare cases.
The classification of engineering alloy steels is as follows.
- Heat-resistant steels are actively used for the production of parts intended for work in the energy sector (for example, components for steam turbines), and they are also used to make especially important fasteners. Chromium, molybdenum, and vanadium are used as alloying additives. Heat-resistant steels refer to medium-carbon, medium-alloy, pearlitic steels.
- Improved steels (from the categories of medium-carbon, low- and medium-alloyed) steels, in the production of which hardening is used, are used for the manufacture of heavily loaded parts that experience variable loads. They differ in sensitivity to stress concentration in the workpiece.
- Case-hardened steels (from the categories of low-carbon, low- and medium-alloyed) steels, as the name suggests, are subject to carburization followed by hardening. They are used for the manufacture of all kinds of gears, shafts and other parts similar in purpose.
Dependence of the thickness of the cemented layer on temperature and processing time
The classification of construction alloy steels implies their division into the following types:
- Bulk - low-alloy steel in the form of pipes, shaped and sheet products.
- Bridge construction - for road and railway bridges.
- Shipbuilding cold-resistant, normal and high-strength - well resistant to brittle fracture.
- Shipbuilding cold-resistant high strength - for welded structures that will operate in low temperature conditions.
- For hot water and steam - operating temperatures up to 600 degrees are allowed.
- Low-cut, high-strength - used in aviation, sensitive to stress concentration.
- Increased strength using carbonitrite hardening, creating a fine-grained steel structure.
- High strength using carbonitrite hardening.
- Strengthened by rolling at a temperature of 700-850 degrees.
Application of tool alloy steels
Tool alloy steel is widely used in the production of various tools. But in addition to its obvious superiority over carbon steel in terms of hardness and strength, alloy steel also has a weak side - higher fragility. Therefore, such steels are not always suitable for tools that are actively exposed to shock loads. Nevertheless, in the production of a huge range of cutting, impact-stamping, measuring and other tools, alloy tool steels remain indispensable.
Separately, we can note high-speed steel, the distinctive features of which are extremely high hardness and red resistance up to a temperature of 600 degrees. Such steel is able to withstand heat at high cutting speeds, which allows you to increase the speed of metalworking equipment and extend its service life.
A separate category includes alloyed structural steels, endowed with special properties: stainless, with improved electrical and magnetic characteristics. Depending on what elements, as well as in what quantities, are predominantly contained in them, they can be chromium, nickel, chromium-nickel-molybdenum. They are also divided into three-, four- and more-component ones according to the number of alloying additives they contain.
Classification by purpose
Each steel, depending on what it is created for, can necessarily be classified into one of the following categories:
- Structural.
- Instrumental.
- Special purpose with special properties.
The most numerous class is structural steels, designed to create a variety of building structures, instruments, and machines. Structural grades are divided into upgradeable, cemented, spring-spring, and high-strength.
Tool steels are differentiated depending on the tool for which they are produced: cutting, measuring, etc. It goes without saying that the influence of alloying elements on the properties of steel in this group is also great.
Special steels have their own division, which includes the following groups:
- Stainless (aka corrosion-resistant).
- Heat resistant.
- Heat resistant.
- Electrical.
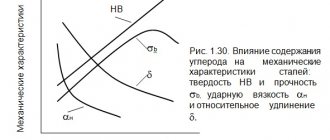
Alloying elements and their influence on the properties of steels
The marking of alloy steels indicates what additives it contains, as well as their quantitative value. But it is also important to know exactly what effect each of these elements has on the properties of the metal separately.
Chromium
The addition of chromium increases corrosion resistance, increases strength and hardness, and is the main component in the creation of stainless steel.
Nickel
The addition of nickel increases the ductility, toughness and corrosion resistance of steel.
Titanium
Titanium reduces the graininess of the internal structure, increasing strength and density, improving machinability and corrosion resistance.
Vanadium
The presence of vanadium reduces the graininess of the internal structure, which increases fluidity and tensile strength.
Molybdenum
The addition of molybdenum makes it possible to improve hardenability, increase corrosion resistance and reduce brittleness.
Tungsten
Tungsten increases hardness, prevents grains from expanding when heated, and reduces brittleness when tempered.
Silicon
At contents of up to 1-15%, silicon increases strength while maintaining toughness. As the percentage of silicon increases, magnetic permeability and electrical resistance increase. This element also increases elasticity, corrosion resistance and oxidation resistance, but also increases fragility.
Cobalt
The introduction of cobalt increases impact resistance and heat resistance.
Aluminum
The addition of aluminum improves scale resistance.
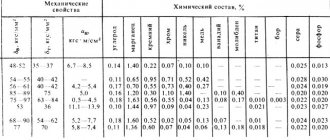
Table of purpose of some types of steel
Separately, it is worth mentioning impurities and their effect on the properties of steels. Any steel always contains technological impurities, since it is extremely difficult to completely remove them from the steel composition. These types of impurities include carbon, sulfur, manganese, silicon, phosphorus, nitrogen and oxygen.
Carbon
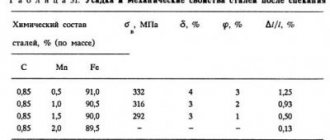
has a very significant effect on the properties of steel. If it is contained up to 1.2%, then carbon helps to increase the hardness, strength, and yield strength of the metal. Exceeding the specified value contributes to the fact that not only strength, but also ductility begins to deteriorate significantly.
Manganese
If the amount of manganese does not exceed 0.8%, then it is considered a technological impurity. It is designed to increase the degree of deoxidation and also counter the negative effects of sulfur on steel.
Sulfur
When the sulfur content exceeds 0.65%, the mechanical properties of steel are significantly reduced, we are talking about a decrease in the level of ductility, corrosion resistance, and impact strength. Also, high sulfur content negatively affects the weldability of steel.
Phosphorus
Even a slight excess of phosphorus content above the required level is fraught with an increase in brittleness and fluidity, as well as a decrease in the toughness and ductility of steel.
Nitrogen and oxygen
When certain quantitative values in the steel composition are exceeded, inclusions of these gases increase brittleness and also contribute to a decrease in its endurance and toughness.
Hydrogen
Too much hydrogen content in steel leads to increased brittleness.
Aluminum alloying
Used in the form of wrought or cast alloys. Alloyed metals based on it are compounds with copper, manganese or magnesium (duralumins and others), the latter are compounds with silicon, the so-called silumins, while all their possible variants are alloyed with Cr, Mg, Zn, Co, Cu, Si.
Copper increases its ductility; silicon – fluidity and high-quality casting properties; chromium, manganese, magnesium - improve strength, technological properties of workability and corrosion resistance. Also, B, Pb, Zr, Ti, Bi can be used as alloying components that contribute to resistance to aging and aggressive operating conditions.
Iron is an undesirable component, but is used in small quantities to make aluminum foil. Silumins are used for casting critical parts and housings in mechanical engineering
Duralumins and aluminum-based stamping alloys are important raw materials for the manufacture of hull elements, including power structures, in aircraft construction, shipbuilding and mechanical engineering
Alloy metals are used in all areas of industry as those that have improved mechanical and technological characteristics in comparison with the original material. The range of alloying elements and the capabilities of modern technologies make it possible to produce various modifications that expand the possibilities in science and technology.
Marking of alloy steels
The category of alloyed steels includes a wide variety of steels, which necessitated the need to systematize their alphanumeric designations. The requirements for their marking are specified by GOST 4543-71, according to which alloys endowed with special properties are indicated by markings with a letter in the first position. By this letter it is possible to determine that the steel, by its properties, belongs to a certain group.
An example of deciphering alloy steel markings
So, if the marking of alloy steels begins with the letters “F”, “X” or “E” - we have an alloy of the stainless, chromium or magnetic group. Steel, which belongs to the stainless chromium-nickel group, is designated by the letter “I” in its marking. Alloys belonging to the category of ball bearing and high-speed tool alloys are designated by the letters “Ш” and “Р”.
Steels classified as alloyed may belong to the category of high-quality, as well as especially high-quality. In such cases, the letter “A” or “W” is placed at the end of their mark, respectively. Steels of ordinary quality do not have such designations in their markings. Alloys that are produced by the rolling method also have a special designation. In this case, the marking contains the letter “N” (hard-worked rolled steel) or “TO” (heat-treated rolled steel).
The exact chemical composition of any alloy steel can be found in regulatory documents and reference literature, but the ability to understand its markings also allows one to obtain such information. The first figure allows you to understand how much carbon (in hundredths of a percent) alloy steel contains. After this number, the brand lists the letter designations of alloying elements that are additionally contained.
Designation of alloying elements in steel markings
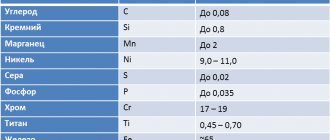
After each such letter the quantitative content of the specified element is indicated. This content is expressed in whole fractions. There may not be any number after the letter indicating the element. This means that its content in steel does not exceed 1.5%. State standard 4543-71 regulates the designation of alloying additives included in alloy steel: A - Nitrogen, B - Niobium, C - Tungsten, G - Manganese, D - Copper, K - Cobalt, M - Molybdenum, N - Nickel, P - Phosphorus, P - Boron, S - Silicon, T - Titanium, C - Zirconium, F - Vanadium, X - Chrome, Yu - Aluminum.
Use of alloy steels
Today it is difficult to find an area of life and activity in which alloy steel would not be used. Almost any tool is made from tool and structural steels: cutters, milling cutters, dies, measuring devices, gears, springs, pendants, braces and much more. Stainless alloy steels are actively used in everyday life; they are used to make dishes, cases and other elements of many types of household appliances.
Due to their high cost, alloy steels are used only for the production of the most critical structures and parts, where products made from other metals simply cannot perform the tasks assigned to them.
Vanadium influence
Vanadium is more often used as a component of complex alloying. Its presence gives alloy steels a more uniform and favorable structure, which changes little even with heat treatment. In addition, vanadium stabilizes the γ-phase, which increases the resistance of steel to shear stress (as is known, it is during shear deformations that metals have the lowest strength).
Vanadium has virtually no effect on the hardness of steel; this is especially noticeable for structural steels, which contain less carbon than tool steels. In complex alloy steels, vanadium increases heat resistance, which increases their resistance to brittle fracture. In this sense, the effect of vanadium is opposite to that of molybdenum. A feature of the heat treatment of alloy steels containing vanadium is the impossibility of performing high tempering after hardening, since the subsequent ductility of the steel is reduced. Therefore, in steels intended for the manufacture of large parts or forgings, the percentage of vanadium is limited to 3..4%.