Catalog
- home
- Technical information
- Metals and alloys
- The influence of chemical elements on the properties of steel and cast iron
Become.
With an increase in carbon content (Fig. 1.30), hardness and strength increase, ductility decreases, cutting processing improves, hardenability increases, but the weldability of steel worsens. The higher the dispersion (smaller crystals) of ferrite and cementite, the higher the hardness and strength.
Harmful impurities for steel are S, P, O, H, N. Sulfur S impairs ductility and toughness; steel becomes brittle at high temperatures (red brittleness), so sulfur in steels should be less than 0.03%. In the presence of sulfur in the alloy, FeS eutectic is created at the edges of the grains, which melts at temperatures above 985 ° C, so cracks form along the grain boundaries and the metal is destroyed.
The presence of phosphorus P in steel leads to cold brittleness (cracks appear already at room temperature and, especially, intensely at negative temperatures), and the ductility and toughness of the alloy deteriorate. High-quality steels should contain less than 0.03% phosphorus.
Manganese Mn deoxidizes steel and neutralizes the harmful effects of sulfur S. increases the strength and wear resistance of steel.
Silicon Si increases the elasticity and strength of steel, increases the yield strength, which reduces the possibility of cold stamping and metal heading.
Cast iron
The microstructure of cast iron (Table 14) depends on the cooling rate of the metal: with rapid cooling there will be white cast iron (carbon is in a chemically bound state in the form of cementite and ledeburite), and with slow cooling there will be gray cast iron (carbon is in the form of graphite).
Table 1.4 Grades and mechanical characteristics of cast iron.
| Groups of cast irons | Cast iron grades | sb, MPa | NV | d, % |
| Gray | SCH 10 | 100 | 120…150 | |
| SCH 15 | 150 | 130…241 | ||
| ……… | ……… | …….. | ||
| SCh 35 | 350 | 179…290 | ||
| High strength | HF 35 | 350 | 140…170 | 22 |
| HF 40 | 400 | 140…202 | 15 | |
| ……… | ………. | …….. | ……… | |
| HF 100 | 1000 | 270…360 | 2 | |
| Malleable | CC 30-6 | ³ 300 | £ 163 | 6 |
| CC 33-8 | ³ 330 | £ 163 | 8 | |
| CC 37-12 | ³ 370 | £ 163 | 12 | |
| ……. | ……. | …… | ||
| CC 63-2 | ³ 630 | £ 269 | 2 |
Silicon Si promotes the graphitization of cast iron and improves its casting properties. Gray cast iron contains 0.8 ... 4.5% Si.
Manganese Mn promotes the bleaching of cast iron, but a Mn content of up to 1.2% is beneficial because the hardness and strength of cast iron increases.
Phosphorus P increases the fluidity of cast iron, so its content up to 0.4% is permissible, but critical cast iron castings contain less than 0.15% phosphorus, because As its content increases, the fragility of cast iron increases.
Sulfur S makes graphitization difficult, increases brittleness and impairs the fluidity of cast iron, so sulfur in cast iron should be no more than 0.1%.
Gray cast irons are divided into modified, high-strength and malleable (Table 1.4.).
In gray cast irons, graphite has a lamellar shape, in high-strength cast irons it has a spherical shape, and in malleable cast irons it has a flake shape.
Examples of cast iron designations:
SCh25 GOST 1412-85, VC 50 GOST 7293-85.
Ductile iron
It has a ferritic or pearlitic structure (see Fig. 23), and is a type of gray cast iron modified with magnesium. Simultaneously with it or a little later, ferrosilicon is introduced into the liquid cast iron. As a result, small inclusions of spherical graphite are obtained (see Fig. 25, 6). This cast iron has increased strength compared to conventional gray cast iron. Depending on the tensile strength (σв) and relative elongation (δ), high-strength cast irons (GOST 7293-79) are divided into the following grades (the numerical values of hardness NV are indicated in parentheses): VCh 38-17 (140-170), VCh 42 -12 (140-200), HF 45-5 (160-220), HF 50-2 (180-260), HF 60-2 (200-280), HF 70-3 (229-275), HF 80 -3 (220-300), HF 100-4 (302-369). HF 120-4 (302-369).
The mechanical properties of high-strength cast iron make it possible to use it for the manufacture of machine parts operating under severe conditions, instead of forgings or castings of steel. High-strength cast iron is used to make parts of rolling mills, forging and pressing equipment, steam turbines (guide vanes), tractors, cars (crankshafts, pistons), etc. For example, the crankshaft of a Volga passenger car is made of high-strength cast iron of the following composition : 3.4-3.6% C; 1.8-2.2% Si; 0.96-1.2% MP; 0.16-0.30% Cg; <0.01% S; <0.06% P and 0.01-0.03% Mg. The low content of sulfur and phosphorus and small limits for the content of other chemical elements are ensured by the fact that such cast iron is smelted not in a cupola, but in an electric furnace. After heat treatment, the mechanical properties of cast iron are very high: σв = 620-650 MPa, δ = 8-12% and hardness HB = 192-240.
What is cast iron?
Cast iron is an alloy of iron and carbon. The carbon component can vary between 2.4 - 4.5%. The amount and state of carbon present influences the properties of cast iron and its applications:
- White cast iron. The carbon in it is in the form of iron carbide, which provides excellent hardness. The structure of this alloy is fine-grained and difficult to process. At the break it is silvery-white. Typically used for steel smelting.
- Grey. Carbon is found in a free state in the form of graphite. Its small plates increase fragility and reduce strength. This alloy has excellent casting properties and is easy to process.
- Highly durable. Graphite inclusions are spheroidal in shape. Decent strength, excellent casting characteristics.
- Malleable. Made from white cast iron. Graphite inclusions in the form of flakes. Viscous and fusible.
An important parameter characterizing the influence of carbon and the most important components in the alloy composition is the carbon equivalent of cast iron, determined by a special formula.
Manufacturing technologies
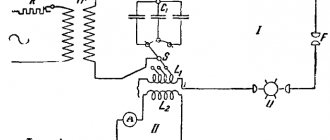
As you know, cast iron is produced in special blast furnaces. The main raw material for its production is iron ore. The manufacturing process consists of the reduction of iron ore oxides and the resulting production of another material - cast iron. For its production, fuels such as coke, thermoanthracite, and natural gas are used.
To produce one ton of pig iron, about 550 kilograms of coke and approximately a ton of water are required. The volume of ore loaded into the furnace will depend on the iron content in it. As a rule, ore is used, which contains at least 70% iron. The thing is that it is not economically feasible to use a lower concentration.
The first stage in the production of cast iron is its smelting. Ore is poured into the blast furnace, and then coking coal, which is necessary to pump and maintain the required temperature inside the furnace shaft. During combustion, these components take an active part in the ongoing chemical reactions as iron reducers.
Meanwhile, flux is immersed in the furnace, which acts as a catalyst. By accelerating the melting of rocks, it thereby supports the rapid release of iron. It is important to know that before loading into the furnace, the ore undergoes the necessary pre-treatment. It is crushed in a crushing plant because smaller particles melt faster. It is then washed to remove non-metal particles. Next, the raw material is fired, as a result of which sulfur and other foreign components are extracted from it.
At the second stage of production, natural gas is supplied through special burners into the filled and ready-to-use furnace. Coke is involved in heating the raw materials. Carbon is released, which combines with oxygen to form an oxide. It, in turn, promotes the recovery of iron from ore.
As the volume of gas in the furnace increases, the rate of the chemical reaction decreases. It may even stop completely when a certain gas ratio is reached. Carbon penetrates the alloy and combines with iron to form cast iron. Unmelted elements remain on the surface and are soon removed. Such waste is called slag. It is used to make other materials.
First stage of production
Iron smelting occurs as follows. First of all, ore is poured into the furnace, as well as coking coal grades, which serve to pump and maintain the required temperature inside the furnace shaft. In addition, during the combustion process, these products actively take part in the ongoing chemical reactions as iron reducers.
At the same time, flux is loaded into the furnace, serving as a catalyst. It helps the rocks melt faster, which promotes the rapid release of iron.
It is important to note that the ore undergoes special pre-treatment before loading into the furnace. It is crushed in a crushing plant (small particles melt faster). Afterwards it is washed to remove particles that do not contain metal. After which the raw material is fired, due to this, sulfur and other foreign elements are removed from it.
Second stage of production
Natural gas is supplied to the loaded and ready-to-use furnace through special burners. Coke heats the raw material. This releases carbon, which combines with oxygen to form an oxide. This oxide subsequently takes part in the reduction of iron from the ore. Note that as the amount of gas in the furnace increases, the rate of the chemical reaction decreases, and when a certain ratio is reached, it stops altogether.
Excess carbon penetrates the melt and combines with iron, ultimately forming cast iron. All those elements that have not melted end up on the surface and are eventually removed. This waste is called slag. It can also be used to produce other materials. The types of cast iron obtained in this way are called foundry and conversion.
Cast iron composition in percentage
Foundry technologist offers cooperation: * I offer all legal entities and individuals interested in obtaining high-quality castings consultations on solving problems in foundry production. Consultations on foundry technology: * reducing losses from defects, reducing gas and clogged sinks * increasing labor productivity by 5- 15%, without investing costs, by identifying losses and changing the chain of technological flows for manufacturing castings * selection of equipment for foundry * assistance in finding manufacturers of pattern equipment for foundry - negotiated individually. *Assistance in assessing the condition of foundry equipment. *Assistance in preparing production when mastering castings. *Advertising of casting manufacturers, foundry equipment, materials and devices for foundry production on this website. Contacts: tel:89080593100 Evgeniy e-mail
How to distinguish cast iron from steel
At scrap metal collection points, cast iron is accepted at a low price, since it is fragile, difficult to process, and removing harmful impurities from it is not an easy task. Experts shared with us tips on how to independently distinguish cast iron from other metals:
- by sound (if you hit steel with any object, the sound comes out very loud);
- by strength;
- on magnetic properties.
Cast iron can be easily distinguished from iron. Iron rusts quickly, it is light silver in color and ductile. But it is more difficult to distinguish cast iron from steel, due to their similar composition; they are externally similar to each other, but the characteristics of the alloys are different from each other.
Steel is easier to process and is not afraid of impacts. There is an opinion that a magnet will help distinguish cast iron from steel. This is correct to some extent, because the magnetic properties of the alloy depend on its composition.
What is
Cast iron is an iron-based alloy.
Belongs to the group of ferrous metals. Ferrous metals are iron, alloys based on it (steel, cast iron, ferroalloys), manganese. According to some classifications, chromium is included in the group.
In terms of composition, cast iron is a conglomerate of iron, carbon, plus other metals. The steel formula could contain the same basic components.
The difference between these alloys is the amount of carbon. If it is less than 2.14%, it is steel. More - cast iron.
Other components are alloys and impurities (sulfur, silicon, phosphorus, manganese).
Carbon in the structure of cast iron is represented by inclusions of graphite or cementite (iron carbide, formula – Fe3C).
You can distinguish cast iron from steel visually. Steel is lighter and shiny, cast iron is dark matte.
Advantages and disadvantages
Cast iron, like any material, has positive and negative sides.
The advantages of cast iron include:
- Carbon in cast iron can be in different states. Therefore, this material can be of two types (gray and white).
- Certain types of cast iron have increased strength, so cast iron is sometimes placed on the same line as steel.
- Cast iron can maintain temperature for quite a long time. That is, when heated, the heat is evenly distributed throughout the material and remains in it for a long time.
- In terms of environmental friendliness, cast iron is a clean material. Therefore, it is often used to make dishes in which food is subsequently prepared.
- Cast iron is resistant to acid-base conditions.
- Cast iron has good hygiene.
- The material has a fairly long service life. It has been noticed that the longer cast iron is used, the better its quality.
- Cast iron is a durable material.
- Cast iron is a harmless material. It is not capable of causing even slight harm to the body.
The disadvantages of cast iron include:
- Cast iron will rust if it is exposed to water for a short time.
- Cast iron is an expensive material. However, this minus is justified. Cast iron is very high quality, practical and reliable. Items made from it are also high quality and durable.
- Gray cast iron is characterized by low ductility.
- White cast iron is characterized by brittleness. It is mainly used for smelting.
Malleable iron
Malleable cast iron is a conventional name for cast iron that is more ductile compared to gray cast iron. Malleable cast iron is never forged. Ductile iron castings are produced by long-term annealing of white cast iron castings with a pearlite-cementite structure. The thickness of the casting walls should not exceed 40-50 mm. During annealing, white cast iron cementite disintegrates to form flake-shaped graphite (see Fig. 25, c). For castings with a wall thickness greater than 50 mm, undesirable flake graphite will form during annealing.
Depending on the structure of the metal base, a distinction is made between ductile ferritic cast iron and ductile pearlitic cast iron. Ferritic malleable cast irons are obtained from white cast irons smelted by the duplex process and containing 2.4-2.8% C; 0.8-1.4% Si; 0.3-0.4% MP; 0.08-0.1% S, 0.2% R. To protect against oxidation during annealing, white cast iron castings are placed in special metal boxes and covered with sand, steel filings or fireclay. Annealing of white cast iron consists of slow heating (20-25 hours) to a temperature of 950-1000°C and long exposure (10-15 hours) at this temperature. During the holding process, the first stage of graphitization occurs, which consists of the decomposition of eutectic and excess secondary cementite, which is present in small quantities at this temperature. By the end of holding, the first stage of graphitization ends and the cast iron consists of austenite and annealed carbon inclusions. Then the temperature is reduced to 720-740°C and the cast iron is again kept for 25-30 hours (Fig. 26, mode 1). At this time, the second stage of graphitization occurs, during which the perlite cementite decomposes. Ferritic malleable cast iron is also called black-core due to the type of fracture, which, due to the large number of graphite inclusions in the ferrite base, has a dark matte color.
Rice. 26. Annealing white cast iron
Pearlitic malleable cast irons are obtained from white cast irons, smelted primarily in cupola furnaces. For this purpose, white cast iron must have the following chemical composition: 2.8-3.4% C; 0.5-0.8% Si; 0.4-0.5% MP; 0.2% P and 0.12% S. To reduce the carbon content, annealing is performed in an oxidizing environment. To do this, the castings are covered with scale or crushed iron ore. The annealing mode consists of heating to a temperature of approximately 1000°C, long-term exposure at this temperature (the first stage of graphitization) and continuous slow cooling to room temperature (Fig. 26, mode 2). With such annealing, a significant part of the carbon burns out, and complete decarbonization is observed in the surface layer up to 1.5-2.0 mm deep. Therefore, when fractured, cast iron turns out to be light-colored and is called light-hearted. Pearlitic malleable cast irons have less use than ferritic malleable cast irons.
Depending on the tensile strength (σв) and relative elongation (δ), malleable cast iron (GOST 1215-79) is divided into the following grades (the numerical values of hardness HB are indicated in parentheses): KCh 30-6 (163), KCh 33-8 (163), CC 35-10 (163), CC 37-12 (163) – ferritic black-hearted and CC 45-6 (241), CC 50-4 (241), CC 56-4 (269), CC 60- 3 (269), CC 63-2 (269) – light-heart pearlite.
Malleable cast iron is widely used in automotive, agricultural and textile engineering. High-strength parts are made from it, capable of withstanding repeated variable and shock loads and operating under conditions of increased wear, such as the rear axle housing, brake pads, hubs, pins of cutting devices of agricultural machines, gears, hook chains, etc. Widespread use of malleable cast iron, which occupies an intermediate position in mechanical properties between gray cast iron and steel, is due to the better casting properties of the original white cast iron compared to steel, which makes it possible to obtain castings of complex shapes. Malleable cast iron is characterized by fairly high anti-corrosion properties and works well in an environment of moist air, flue gases and water.