A striking example of allotropy is iron, which forms, depending on temperature, four main allotropic modifications, which are called: α-Fe, β-Fe, γ-Fe, δ-Fe.
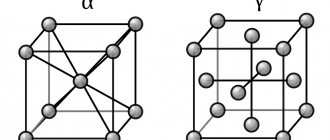
Allotropic forms α-Fe - alpha iron, β-Fe - beta iron and δ-Fe - sigma iron have a crystal lattice in the shape of a centered cube (Fig. 1, a). The allotropic form of γ-Fe - gamma iron has a crystal lattice in the shape of a cube with centered faces (Fig. 1, b).
The transition of iron from one form to another - when cooled, it releases heat, and when heated, it absorbs heat. This is noted on the graphs of cooling or heating of iron. In Fig. Figure 2 shows a schematic graph of the cooling of pure iron.
Photo of iron crystal lattices
The crystal lattice is the spatial arrangement of atoms or ions in a crystal.
The points of the crystal lattice at which atoms or ions are located are called lattice nodes. Crystal lattices are divided into molecular, atomic, ionic and metallic.
On this curve, during transitions from one allotropic form to another, areas of constant temperatures are observed, namely: at t = 1535° - solidification of iron with the formation of δ-Fe;
photo of iron crystal lattices
at t = 1390°—transition δ-Fe - γ-Fe;
at t=898° - transition γ-Fe - β-Fe;
at t=775° - transition β-Fe - α-Fe.
When heating iron, the transformations occur in the reverse order, and the transition β -Fe - > γ-Fe occurs at t = 910°. In γ-Fe, the atoms are located more closely than in β-Fe, therefore the transition of γ-Fe to β-Fe is accompanied an increase in volume, and vice versa, the transition of β-Fe to γ-Fe is accompanied by a decrease in volume. The γ-Fe phosphorus is not magnetic.
The α-Fe form is magnetic. The β-Fe form is not magnetic, but has the same crystal lattice as the magnetic α-Fe form - therefore, metallographically, the β-Fe form is identified with the α-Fe form and I conditionally unite both forms under the same name α-Fe.
The main role in the technological processes of hot mechanical and thermal processing of iron-carbon alloys is played by: α-Fe and γ-Fe.
Rice. 2 Iron cooling curve
The property of γ-Fe to form solid solutions with carbon is important for heat treatment. The highest solubility of carbon in γ-Fe (up to 1.7%) is observed at t=1130°. With increasing and decreasing temperature from t = 1130°, the solubility of carbon in γ-Fe decreases.
The solid solution of carbon and other elements in γ-Fe is called austenite. α-Fe does not form stable solid solutions with carbon, like austenite. The solubility of carbon in α-Fe is negligible. Solid solutions of small amounts of C and other elements in α-Fe are called ferrite.
In addition to solid solutions of carbon in iron, iron-carbon alloys contain a chemical compound of iron and carbon - iron carbide Fe3C, which is called cementite. Cementite contains C - 6.67%. Austenite and ferrite are distinguished by ductility, ferrite, in addition, is soft. Cementite is extremely hard and brittle.
Atomic-crystalline structure of metals
The internal structure of metals and their characteristics determine their physical and chemical properties. Electrons in the outer orbits of atoms are weakly bound to the nucleus and have a negative charge. When there is a potential difference, electrons migrate to the positive pole, creating an electric current. This physical phenomenon causes electrical conductivity.
The crystalline structure is characteristic of metals and their alloys in the solid phase state. Atoms are arranged in a certain three-dimensional structure called a crystal lattice. The number of atoms at the vertices and faces of this structure, as well as the distance between them, determines such physical properties of the metal as electrical and thermal conductivity, viscosity, fluidity, etc. The crystal structure of metals and alloys can be of two types:
- The interatomic distance is the same in all directions. This is the so-called isotropic structure. Moreover, the physical properties of the crystal are also the same in all directions.
The interatomic distance horizontally and vertically is different. Such a crystal is called anisotropic, and its physical parameters vary depending on the direction.
Atomic crystal structure of metals
In a real piece of metal, composed of many isolated crystalline fragments, the atomic-crystalline structure belongs to the third type - quasi-isotropic. On average, the properties of such a piece are close to isotropic. When building a crystal lattice, some atoms do not fall into place, are displaced or lost. In this case, they speak of defects in the crystalline structure of metals. Structural defects negatively affect the properties of the product, especially if it must be a single crystal, as, for example, in electronics, laser technology and other high-tech industries.
APPLICATION
Iron ring
Iron is one of the most used metals, accounting for up to 95% of global metallurgical production. Iron is the main component of steels and cast irons - the most important structural materials. Iron can be part of alloys based on other metals - for example, nickel. Magnetic iron oxide (magnetite) is an important material in the production of long-term computer memory devices: hard drives, floppy disks, etc. Ultrafine magnetite powder is used in many black and white laser printers mixed with polymer granules as a toner. This uses both the black color of the magnetite and its ability to stick to the magnetized transfer roller. The unique ferromagnetic properties of a number of iron-based alloys contribute to their widespread use in electrical engineering for magnetic cores of transformers and electric motors. Iron(III) chloride (ferric chloride) is used in amateur radio practice for etching printed circuit boards. Ferrous sulfate heptate (ferrous sulfate) mixed with copper sulfate is used to combat harmful fungi in gardening and construction. Iron is used as an anode in iron-nickel batteries and iron-air batteries. Aqueous solutions of ferrous and ferric chlorides, as well as its sulfates, are used as coagulants in the purification processes of natural and waste water in the water treatment of industrial enterprises.
Iron - Fe
| Molecular weight | 55.85 g/mol |
| origin of name | Possibly Anglo-Saxon origin |
| IMA status | valid, first described before 1959 (before IMA) |
Atomic crystal lattice
Substances with an atomic crystal lattice, as a rule, have strong covalent bonds in their nodes consisting of atoms themselves. A covalent bond occurs when two identical atoms share fraternal electrons with each other, thus forming a common pair of electrons for neighboring atoms. Because of this, covalent bonds bind atoms tightly and evenly in a strict order - perhaps this is the most characteristic feature of the structure of the atomic crystal lattice. Chemical elements with similar bonds can boast of their hardness and high melting point. Chemical elements such as diamond, silicon, germanium, and boron have an atomic crystal lattice.
Iron, properties of the atom, chemical and physical properties.
Fe 26 Iron
55.845(2) 1s2 2s2 2p6 3s2 3p6 3d6 4s2
Iron is an element of the periodic system of chemical elements of D.I. Mendeleev with atomic number 26. It is located in the 8th group (according to the old classification - a secondary subgroup of the eighth group), the fourth period of the periodic system.
Iron atom and molecule. Iron formula. Structure of the iron atom
Isotopes and modifications of iron
Properties of iron (table): temperature, density, pressure, etc.
Physical properties of iron
Chemical properties of iron. Interaction of iron. Chemical reactions with iron
Getting iron
Application of iron
Table of chemical elements D.I. Mendeleev
Types of crystal lattices
The distance between neighboring atoms is called the lattice parameter; for different metals it is 2 - 6 angstroms. There are three main types of crystal lattices:
- Cubic: body-centered - contains nine atoms. Characteristic of iron, chromium, molybdenum, and vanadium.
- Cubic face-centered: already includes 14 atoms. Inherent in copper, gold, lead, aluminum.
- Hexagonal: there are already 17 atoms and they are placed most densely. This is how magnesium, zinc, cadmium and others crystallize.
The unique feature of iron is that up to 910°C it has a body-centered cubic structure, and when heated above this temperature it becomes face-centered.
Additional information:
| 513 | Lattice parameters | 2.866 Å |
| 514 | c/a ratio | |
| 515 | Debye temperature | 460 K |
| 516 | Name of space symmetry group | Im_ 3m |
| 517 | Symmetry space group number | 229 |
| 521 | Crystal grid #2 | γ-iron (austenite) |
| 522 | Lattice structure | Cubic face centered |
| 523 | Lattice parameters | 3.656 Å |
| 524 | c/a ratio | |
| 525 | Debye temperature | |
| 526 | Name of space symmetry group | Fm_3m |
| 527 | Symmetry space group number | 225 |
| 531 | Crystal grid #3 | δ-iron |
| 532 | Lattice structure | Cubic body-centered |
| 533 | Lattice parameters | 2.93 Å |
| 534 | c/a ratio | |
| 535 | Debye temperature | |
| 536 | Name of space symmetry group | Im_ 3m |
| 537 | Symmetry space group number | 229 |
| 900 | additional information | |
| 901 | CAS number | 7439-89-6 |
Metal crystal lattice
The type of bond of a metal crystal lattice is more flexible and ductile than the ionic one, although in appearance they are very similar. Its distinctive feature is the presence of positively charged cations (metal ions) at lattice sites. Between the nodes live electrons that participate in the creation of the electric field; these electrons are also called electric gas. The presence of such a structure of a metal crystal lattice explains its properties: mechanical strength, heat and electrical conductivity, fusibility.
Atomic crystal lattice
The nodes contain atoms, as the name suggests. These substances are very strong and durable , since a covalent bond is formed between the particles. Neighboring atoms share a pair of electrons with each other (or rather, their electron clouds are layered on top of each other), and therefore they are very well connected to each other. The most obvious example is diamond, which has the greatest hardness on the Mohs scale. Interestingly, diamond, like graphite, consists of carbohydrates. Graphite is a very brittle substance (Mohs hardness 1), which is a clear example of how much depends on the type.
Crystal lattices, video
And finally, a detailed video explanation about the properties of crystal lattices.
Author: Pavel Chaika, editor-in-chief of Poznavaika magazine
When writing the article, I tried to make it as interesting, useful and high-quality as possible. I would be grateful for any feedback and constructive criticism in the form of comments on the article. You can also write your wish/question/suggestion to my email [email protected] or Facebook, with respect, the author.
Popular science magazine Poznavaika
This article is available in English - Crystal Lattice in Chemistry.
Metal crystal lattice
Due to the presence of ions at the nodes, the metal lattice may appear to be similar to an ionic lattice. In fact, these are two completely different models, with different properties.
Metal is much more flexible and ductile than ionic, it is characterized by strength, high electrical and thermal conductivity, these substances melt well and conduct electric current well. This is explained by the fact that the nodes contain positively charged metal ions (cations), which can move throughout the structure, thereby ensuring the flow of electrons. The particles move chaotically around their node (they do not have enough energy to go beyond), but as soon as an electric field appears, electrons form a stream and rush from the positive to the negative region.
The metal crystal lattice is characteristic of metals, for example: lead, sodium, potassium, calcium, silver, iron, zinc, platinum and so on. Among other things, it is divided into several types of packaging: hexagonal, body-centered (least dense) and face-centered. The first package is typical for zinc, cobalt, magnesium, the second for barium, iron, sodium, the third for copper, aluminum and calcium.
Thus, on the type of lattice . Knowing the type, you can predict, for example, what the refractoriness or strength of an object will be.
Iron crystal lattice
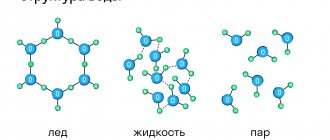
The basic building blocks of solids such as salt and ice are molecules. Each molecule consists of two or more atoms, for example, sodium + chlorine (NaCl), as in table salt, and hydrogen + oxygen, as in ice (H2O). In metals, however, these building blocks are individual metal atoms: iron (Fe) atoms in an iron rod or copper (Cu) atoms in copper wire. Each grain in Figure 1 is what is called a crystal. In a crystal, which consists of atoms, all the atoms are uniformly arranged in layers. As shown in Figure 2, if you draw lines that connect the centers of the atoms, three-dimensional rows of small cubes will fill the entire space occupied by an individual grain. This three-dimensional structure is called a crystal lattice of atoms.
Figure 2 – Crystal lattice of iron
Properties of the iron atom:
200
| Properties of the atom | ||
| 201 | Atomic mass ( molar mass ) | 55.845(2) amu (g/mol) |
| 202 | Electronic configuration | 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2 |
| 203 | Electronic shell | K2 L8 M14 N2 O0 P0 Q0 R0 |
152 pm – high-spin207Ion radius (crystalline)Fe 2+ low spin
(in parentheses the coordination number is indicated - a characteristic that determines the number of nearest particles (ions or atoms) in a molecule or crystal) 208 Van der Waals radius 209 Electrons, Protons, Neutrons 26 electrons, 26 protons, 30 neutrons 210 Family (block) element of the d-family 211 Period in the periodic table table 4212 Group in the periodic table 8th group (according to the old classification - a side subgroup of the 8th group) 213 Emission spectrum
| 400 | Physical properties | |
| 401 | Density | 7.874 g/cm 3 (at 0 °C/20 °C and other standard conditions, state of matter - solid), |
6.98 g/cm 3 (at a melting point of 1538 °C and other standard conditions, the state of the substance is liquid),
6.9 g/cm 3 (at 1589 °C and other standard conditions, state of matter – liquid)
Crystal structure of metals. Types of crystal lattices
When a metal forms a solid structure, all its atoms tend to occupy such positions in space relative to each other that they correspond to a minimum of potential energy. This minimum corresponds to the crystal lattice.
A crystal lattice is understood as a spatial atomic structure that can be obtained if the coordinates of a limited number of its atoms and the vector of their translation in space are known. The specified number of atoms is called the lattice basis, and their positions form the so-called unit cell.
All metals crystallize in three main types of lattices:
- face-centered cubic (fcc);
- body-centered cubic (bcc);
- hexagonal close-packed (hcp).
Due to their crystalline structure, metals have properties such as plasticity, elasticity and metallic luster.
GCC, BCC, GPU lattices
Studying the crystal structure of metals, we will characterize in more detail each type of crystal lattice. Let's start with the HCC. It is shown in the figure below.
As you can see, this lattice is a cube in which atoms are located at its vertices and at the centers of all six faces. Using crystallographic methods, it is easy to show that to obtain such a lattice in space, only four atoms and translation vectors coinciding with the edges of the cube are sufficient.
Examples of metals that crystallize in fcc are aluminum, copper, gold and silver. Iron forms an fcc lattice only at high temperatures.
The bcc lattice is shown below.
We see that it corresponds to a cube, at the vertices and in the center of which there is an atom. Only two atoms are needed to construct a bcc lattice in rectangular Cartesian coordinates. Metals such as vanadium, tantalum, niobium, and tungsten have exactly this crystalline structure.
Finally, the GPU grid. It is shown below in the figure.
This crystal lattice of metals differs from the previous two in that in space it forms not a cube, but a regular hexagonal prism, which consists of six atoms. Elements such as titanium, zirconium, magnesium and cobalt crystallize in this structure.
Note:
115* Temperature and other conditions for the transition of allotropic modifications of iron into each other according to [1]:
— α-iron (ferrite) exists at temperatures below 770 °C and normal conditions (Curie point of iron according to [1] 770 °C),
— β-iron exists in the temperature range from 770 °C to 912 °C and normal conditions,
— γ-iron (austenite) exists in the temperature range from 912 °C to 1394 °C and normal conditions,
— δ-iron exists at temperatures above 1394 °C and normal conditions,
— ε-iron exists at a temperature of several hundred °C and a pressure of more than 10 GPa or at higher pressure and normal conditions.
205* The empirical radius of the iron atom according to [1] and [3] is 126 pm.
206* The covalent radius of iron according to [1] is 132±3 pm (low-spin) and 152±6 pm (high-spin), the covalent radius of iron according to [3] [Russia] is 117 pm.
402* The melting point of iron according to [3] and [4] is 1538.85 °C (1812 K, 2801.93 °F) and 1539 °C (1812.15 K, 2802.2 °F), respectively.
403* The boiling point of iron according to [4] is 2870 °C (3143.15 K, 5198 °F).
407* The specific heat of fusion (enthalpy of fusion ΔHmel) of iron according to [3] and [4] is 13.8 kJ/mol.
408* The specific heat of evaporation (enthalpy of boiling ΔHboiling) of iron according to [4] is 350 kJ/mol.
410* The molar heat capacity of iron according to [3] is 25.14 J/(K mol).
428* The Curie point of iron according to [1] is 770 °C (1043 K, 1418 °F).
Source
Crystal structure
MATERIALS SCIENCE
Substances can be in three states of aggregation: solid, liquid and gaseous. The transition from a solid to a gaseous state is called sublimation.
All metals are crystalline solids.
Each metal is characterized by its own spatial crystal lattice with long-range order in the arrangement of atoms (a certain arrangement of atoms at any distance).
In solids, the order of arrangement of atoms is natural. The arrangement of atoms can be represented in the form of elementary crystalline cells. There are 14 types of gratings in total: 3 types are typical for metals:
1. Volume-centered cubic (BCC);
2. Grane - centered cubic (fcc);
3. Hexagonal close-packed (hcp).
Iron has two types of crystal lattices:
— body-centered cubic: atoms are located in the center of the cube and along its vertices;
— face-centered cubic: atoms are located in the centers of the faces and along the vertices of the cube.
A number of metals change the type of crystal lattice with changes in temperature; this property of metals is called polymorphism (multiformity, allotropy). For iron (Fe) at temperatures up to 911 °C - bcc; from 911 to 1392 °C - fcc; over 1392 °C - bcc.
Metals are made up of large numbers of irregularly shaped crystals called grains. In real metals, the crystal lattice is not ideal. The internal structure of the grain has defects.
Crystal lattice defects:
1. Point (zero-dimensional);
2. Linear (one-dimensional);
3. Surface (two-dimensional);
4. Volumetric (three-dimensional).
The spaces unoccupied by atoms are called “vacancies”. Atoms of other elements can replace atoms or be incorporated into the lattice. The solidity of welded joints is ensured by the appearance of atomic-molecular bonds between the elementary particles of the substances being joined: covalent, ionic, intermolecular, metallic. To connect materials, it is necessary to ensure contact along the joining surface and activate it. Activation energy is transferred to the connected surfaces in the form of heat, elastoplastic deformation, electronic or other type of irradiation. Energy is necessary to break the bonds between the atoms of a substance and the external environment, as well as to transform them into an active state.
The metal bond (lattice) is characterized by sufficient strength and ductility and depends on the type and number of lattice elements.
Volumetric defects include cracks, lack of penetration, lack of fusion, pores, slags (they have significant sizes in 3 dimensions).
Simplified classification of iron-carbon alloys
It is conventionally considered that iron-carbon alloys containing less than 2.14% carbon are steel, and more than 2.14% are cast iron. Alloys include compounds containing less than 50% iron.
Carbon steels contain iron and carbon. To impart special properties, other elements are introduced into steel. Such steels are called alloyed.
General purpose carbon steels are supplied:
group A – with guaranteed mechanical properties (steel is not subjected to heat treatment);
group B - with a guaranteed chemical composition (steel is subjected to heat treatment);
group B - with guaranteed mechanical properties and chemical composition.
Boiling steels (boiling steels) are deoxidized by Mn, semi-quiet steels (ps) are deoxidized by Mn, Si, calm steels (sp) are deoxidized by Mn, Si, Al.
An example of the designation of steel of group A - St.3kp, group B - B St.3sp.
High-quality carbon steels are characterized by a reduced content of harmful impurities - phosphorus and sulfur. In the designation of steels, the numbers indicate the carbon content in hundredths of a percent, for example - Steel 10 contains 0.10% carbon.
Low-carbon steels contain less than 0.25% carbon, medium-carbon steels contain 0.25...0.46%, and high-carbon steels contain more than 0.46%.
Alloying makes it possible to increase strength properties, provide corrosion resistance, heat resistance, etc. Depending on the content of alloying elements, steels are divided into low-alloy (up to 2.5%), medium-alloy (2.5...10%) and high-alloy (more than 10%).
RESERVES AND PRODUCTION
Iron is one of the most common elements in the solar system, especially on the terrestrial planets, in particular on Earth. A significant part of the iron of the terrestrial planets is located in the cores of the planets, where its content is estimated to be about 90%. The iron content in the earth's crust is 5%, and in the mantle about 12%.
Iron
Iron is quite widespread in the earth's crust - it accounts for about 4.1% of the mass of the earth's crust (4th place among all elements, 2nd among metals). In the mantle and earth's crust, iron is concentrated mainly in silicates, while its content is significant in basic and ultrabasic rocks, and low in acidic and intermediate rocks. A large number of ores and minerals containing iron are known. Of greatest practical importance are red iron ore (hematite, Fe2O3; contains up to 70% Fe), magnetic iron ore (magnetite, FeFe2O4, Fe3O4; contains 72.4% Fe), brown iron ore or limonite (goethite and hydrogoethite, respectively FeOOH and FeOOH nH2O ). Goethite and hydrogoethite are most often found in weathering crusts, forming so-called “iron hats”, the thickness of which reaches several hundred meters. They can also be of sedimentary origin, falling out of colloidal solutions in lakes or coastal areas of the seas. In this case, oolitic, or legume, iron ores are formed. Vivianite Fe3(PO4)2·8H2O is often found in them, forming black elongated crystals and radial aggregates. The iron content in sea water is 1·10−5-1·10−8%. In industry, iron is obtained from iron ore, mainly from hematite (Fe2O3) and magnetite (FeO·Fe2O3). There are various ways to extract iron from ores. The most common is the domain process. The first stage of production is the reduction of iron with carbon in a blast furnace at a temperature of 2000 °C. In a blast furnace, carbon in the form of coke, iron ore in the form of agglomerate or pellets, and flux (such as limestone) are fed from above, and are met by a stream of forced hot air from below. In addition to the blast furnace process, the process of direct iron production is common. In this case, pre-crushed ore is mixed with special clay, forming pellets. The pellets are fired and treated in a shaft furnace with hot methane conversion products, which contain hydrogen. Hydrogen easily reduces iron without contaminating the iron with impurities such as sulfur and phosphorus, which are common impurities in coal. Iron is obtained in solid form and is subsequently melted in electric furnaces. Chemically pure iron is obtained by electrolysis of solutions of its salts.
General structure
Metals are solid substances with a crystalline structure. The exception is mercury, a liquid metal. Crystal lattices are metal atoms ordered in a certain way. Each atom consists of a positively charged nucleus and several negatively charged electrons. Metal atoms don't have enough electrons, so they are ions.
A unit of a crystal lattice is an elementary crystal cell, in the conventional nodes and on the faces of which there are positively charged ions. They are held together by metallic bonds that arise due to the random movement of electrons separated from the atoms (due to which the atoms turned into ions).
Rice. 1. Metal connection diagram.
The free movement of electrons determines the electrical and thermal conductivity of metals.
FAST
SEO optimization
Adaptive layout
Repair in the regions
- home
- Construction Materials
- Crystal structure of metals
- Alpha beta sigma gamma iron
A striking example of allotropy is iron, which forms, depending on temperature, four main allotropic modifications, which are called: α-Fe, β-Fe, γ-Fe, δ-Fe.
Allotropic forms α-Fe - alpha iron, β-Fe - beta iron and δ-Fe - sigma iron have a crystal lattice in the shape of a centered cube (Fig. 1, a). The allotropic form of γ-Fe - gamma iron has a crystal lattice in the shape of a cube with centered faces (Fig. 1, b).
The transition of iron from one form to another - when cooled, it releases heat, and when heated, it absorbs heat. This is noted on the graphs of cooling or heating of iron. In Fig. Figure 2 shows a schematic graph of the cooling of pure iron.
Physical properties of iron:
| 115 | Temperature and other conditions for the transition of allotropic modifications into each other* | — α-iron (ferrite) exists at temperatures below 769 °C and normal conditions (Curie point of iron 769 °C), — β-iron exists in the temperature range from 769 °C to 917 °C and normal conditions, — γ-iron (austenite) exists in the temperature range from 917 °C to 1394 °C and normal conditions, — δ-iron exists at temperatures above 1394 °C and normal conditions, |
What are pearlite and eutectoid
Observations show that this transition occurs as follows: upon reaching temperatures GS along the boundaries Observations show that this transition occurs as follows: upon reaching temperatures GS along the boundaries of austenite crystals, the first portions of α - Fe, i.e., ferrite, are released, the amount of which gradually increases.
Since ferrite almost does not dissolve carbon, during the transition γ-Fe -> α-Fe, the carbon concentration in retained austenite gradually increases and can be determined by the GS line depending on temperature. The ferrite separation process continues until the carbon concentration corresponds to point 5, i.e., up to C = 0.83%, and the temperature reaches t = 723°.
At point S, the GS curve intersects with ES, the curve of the limiting solubility of carbon in austenite. Therefore, further saturation of retained austenite with carbon becomes impossible, and subsequent cooling causes the final decomposition of austenite, which occurs at a constant temperature t = 723°.
During this decomposition, the γ-Fe->α-Fe transition is completed, and the carbon released from the iron crystal lattice forms F3C cementite particles. The decomposition of austenite occurs in a confined space within each grain, so the decomposition products (ferrite and cementite) are formed in the form of closely mixed particles, usually in the form of alternating plates of ferrite and cementite.
Scheme of changes in the structure of steels when passing through critical points
This decomposition product of austenite is called pearlite; Since pearlite has a structure similar to eutectic, it is called a eutectoid. The difference between eutectic and eutectoid is that eutectic is formed from a liquid solution, while eutectoid is formed from a solid.
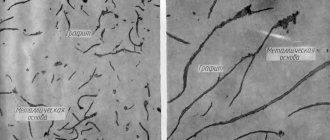
The formation of pearlite begins and ends at a constant t=723°. This is how the ferrite-pearlite structure of steels appears, which, upon further cooling from t = 723°, no longer undergoes any structural changes. The figure shows the microstructures of pure iron and steel at C = 0.15% and at C = 0.6% (magnification 100) after etching the scratched surface of a microsection with a 4% solution of HNO3 in ethyl alcohol.
Rice. 1. - ferrite in pure iron. Rice. 2 Hypoeutectoid steel with C content = 0.15%
In Fig. 1, which shows the microstructure of pure iron, the boundaries between the light ferrite grains are clearly visible. In Fig. Figure 2 shows the microstructure of building steel (C=0.15%); light fields are ferrite, dark areas are pearlite. In Fig. Figure 3 shows the microstructure of engineering steel (C = 0.6%), from which axles, shafts, connecting rods, etc. are made; Most of the section is occupied by pearlite, and ferrite is observed only in the form of a thin mesh. The more carbon, the more pearlite in the steel structure; the composition of pearlite is the same (C = 0.83%). The structure of perlite is usually lamellar (Fig. 4).
Rice. 3 Hypoeutectoid steel with C content = 0.6%. Rice. 4 Eutectoid steel (lamellar pearlite).
Ferrite, as mentioned above, is the softest plastic component of iron-carbon alloys; cementite, which is part of pearlite, is the hardest and most brittle, therefore, with increasing carbon content, the strength and hardness of steel increases, but ductility and toughness decrease
In order for construction steel to be sufficiently ductile, the amount of perlite in it should not exceed 25%, which corresponds to a carbon content of up to 0.2%.
In those parts that require greater strength and hardness, but lower ductility and toughness are acceptable (machine parts), steels with a large amount of pearlite, with a C content of up to 0.6%, are used. In the construction industry, such steels are used, for example, for the manufacture of shovels and supporting parts of bridge trusses.
ORIGIN
Origin telluric (terrestrial) iron is rarely found in basaltic lavas (Uifak, Disko Island, off the western coast of Greenland, near Kassel, Germany). At both locations, pyrrhotite (Fe1-xS) and cohenite (Fe3C) are associated with it, which is explained by both the reduction by carbon (including from the host rocks) and the decomposition of carbonyl complexes such as Fe(CO)n. In microscopic grains, it has more than once been established in altered (serpentinized) ultrabasic rocks, also in paragenesis with pyrrhotite, sometimes with magnetite, due to which it arises during reduction reactions. Very rarely found in the oxidation zone of ore deposits, during the formation of swamp ores. Findings have been recorded in sedimentary rocks associated with the reduction of iron compounds with hydrogen and hydrocarbons.
Almost pure iron was found in lunar soil, which is associated with both meteorite falls and magmatic processes. Finally, two classes of meteorites - stony-iron and iron - contain natural iron alloys as a rock-forming component.