Rimoyt.com
Phases and structural components of iron-carbon alloys
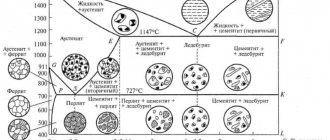
The main components on which the structure and properties of iron-carbon alloys depend are iron and carbon. Pure iron is a silver-white metal with a melting point of 1539 °C. Iron has two polymorphs: alpha () and gamma () . alpha modification exists at temperatures below 911 °C and above 1392 °C; gamma iron – at a temperature of 911-1392 °C. Depending on the temperature and carbon concentration in iron-carbon alloys (steels and cast irons), the following solid phases are formed: ferrite, austenite, cementite, graphite.
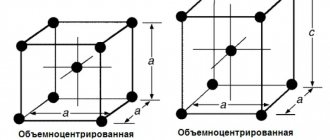
1. Ferrite (F) is a solid solution of intercalated carbon in alpha iron. Alpha iron has a bcc structure that is stable up to 911 °C. The highest solubility of carbon in alpha iron is 0.02% at 727 °C. As the temperature decreases, the solubility of carbon also decreases, and at room temperature it is 0.005% by weight. For this reason, ferrite is called technically pure iron; it has low hardness (HB = 80-100) and strength (tensile strength = 250 MPa), but high ductility (relative elongation up to 50%, relative contraction up to 80%). At temperatures from 1392 °C to 1539 °C, iron also has a bcc structure - it is delta iron. The interstitial solid solution of carbon in delta iron is called high temperature ferrite .
2. Austenite (A) is a solid solution of interstitial carbon in gamma iron. Austenite has an fcc structure. In iron-carbon alloys, austenite can only exist at high temperatures. In gamma iron, carbon dissolves much better than in alpha iron; the maximum solubility of carbon in gamma iron is 2.14% and is observed at a temperature of 1147 ° C. As the temperature decreases, the solubility of carbon decreases to 0.8% at 727 °C. Austenite has a hardness HB = 160-200 and is very ductile (relative elongation 40-50%), observed in steels at temperatures from 727 ° C.
3. Cementite (C) is a chemical compound of iron and carbon (iron carbide Fe3C). Cementite contains 6.67% carbon. The melting point of cementite is about 1600 °C. It is very hard (HB about 800 units), brittle and has virtually no ductility. There are primary, secondary and tertiary cementite. Their differences lie in their origin: - primary cementite is formed from a liquid melt during the crystallization of iron-carbon alloys (CD line), - secondary cementite precipitates from austenite (due to a decrease in the solubility of carbon in austenite with decreasing temperature - line SE) - tertiary cementite precipitates from ferrite with decrease in temperature (due to a decrease in the solubility of carbon in ferrite with decreasing temperature - line PQ) Cementite is an unstable metastable phase. When heated and held for a long time, cementite decomposes into ferrite (alpha iron) and graphite (Fe3C -> 3Fe + C).
4. Graphite is pure carbon with a hexagonal layered structure. Graphite is very soft (HB = 3) and has low strength. In cast iron and graphitized steel it is found in the form of inclusions of various shapes (plate-like, flake-like, spherical). As the shape of graphite inclusions changes, the mechanical and technological properties of the alloy change.
In addition to the four above-mentioned phases, the structure of iron-carbon alloys contains two more independent structural components: pearlite and ledeburite.
5. Perlite (P) – a mechanical mixture of ferrite and cementite containing 0.8% carbon. Pearlite is formed from austenite when it is cooled to a temperature below 727 °C. Thus, pearlite is a eutectoid . Perlite can be lamellar and granular (globular), which depends on the shape of the cementite and determines the mechanical properties of perlite. At room temperature, granular pearlite has a tensile strength of 800 MPa, a relative elongation of 15%, and a hardness of HB = 160.
6. Ledeburite (L) is a mechanical mixture of austenite and cementite (L = A + C), containing 4.3% carbon. Ledeburite is formed from a liquid melt at a temperature of 1147 °C. Thus, ledeburite is inherently eutectic . Ledeburite is formed when a liquid melt solidifies at 1147 °C. Ledeburite has a hardness of HB = 600-700 HB and great brittleness. Ledeburite is observed in the structure of cast iron; in steel it is formed only with a large number of alloying elements and a carbon content of more than 0.7%. When ledeburite is cooled to a temperature of 727 ° C, the austenite included in its composition becomes unstable and disintegrates, turning into pearlite. Thus, at temperatures below 727 °C and up to 20 °C, ledeburite is a mechanical mixture of perlite and cementite.
| Ferrite | Austenite | Cementite | Graphite | Perlite | Ledeburite | |
| Essence | carbon interstitial solid solution in alpha iron | carbon interstitial solid solution in gamma iron | chemical compound of iron and carbon | pure carbon | mechanical mixture of ferrite and cementite | mechanical mixture of austenite and cementite |
| Designation | F or -Fe(C) | A or -Fe(C) | C or Fe3C | G | P = F + C = Fe?(C) + Fe3C | L = A + C = Fe?(C) + Fe3C |
| Hardness HB | 80-100 | 160-200 | 800 | 3 | 160 | 600-700 |
| Carbon content | up to 0.02% | up to 2.14% | 6,67% | 100% | 0,8% | 4,3% |
Phases and structural components of iron-carbon alloys
In addition to the listed structural components, iron-carbon alloys may contain undesirable non-metallic inclusions: oxides, nitrides, sulfides, phosphides - compounds with oxygen, nitrogen, sulfur and phosphorus.
Alloy theory
Pure metals are relatively rarely used in mechanical engineering, since they do not provide the necessary complex of mechanical and technological properties of parts made from them.
Alloys consisting of two or more elements (two metals, such as copper and zinc, or a metal and a non-metal, such as iron and carbon) are widely used. The elements included in the alloy are called components.
Alloys are produced by fusing components, sintering, electrolysis and sublimation. The components included in the alloy, in the liquid state, almost always dissolve in each other, forming a liquid solution. The atoms of such a solution are uniformly mixed with each other. The properties of alloys depend mainly on the interaction of the components during solidification. When alloys solidify, a solid solution, chemical compound or mechanical mixture is formed.
Solid solution. During the transition to the solid state in alloys, the homogeneity of the distribution of atoms of various components, and therefore the property of solubility, is preserved. When an alloy crystallizes, the atoms of the components enter into a single cell of the crystal lattice, so the resulting grains are homogeneous and identical in composition. The solid solution, like pure metal, has a uniform crystal lattice. In the crystal lattice of a pure metal, all nodes are occupied by atoms of one component, and in the lattice of a solid solution - by atoms of the components that make up the alloy. In solid solutions, the solubility of the components is not limited at any quantitative ratio (copper with nickel).
The properties of alloys that form solid solutions change smoothly and differ from the properties of the components from which they are composed. They are distinguished by valuable properties. They are harder and stronger than their constituent components, have good ductility, high electrical resistance that does not change with temperature changes, and increased resistance to corrosion. Due to their high ductility, such alloys are well processed under pressure.
Chemical compound. During crystallization, the components of some alloys can enter into a chemical bond, forming a chemical compound. For example, iron and carbon form the chemical compound Fe3C - iron carbide (cementite); copper with magnesium - Cu2Mg; magnesium with lead - Mg2Pb, etc.
A chemical compound, like a solid solution, has a homogeneous structure. Its crystal lattice includes atoms of both components. However, in the crystal lattice of a chemical compound, unlike a solid solution, the atoms of each component are in a strictly defined quantity and are always located in the same way. For example, the chemical compound of iron with carbon Fe3C always consists of three iron atoms and one carbon atom, the Mg2Pb compound always has two magnesium atoms and one lead atom. Thus, a chemical compound has a constant composition and is expressed by a chemical formula, but the composition of solid solutions varies within wide limits; a solid solution cannot be expressed by a chemical formula. The crystal lattice of a chemical compound differs from the lattices of its constituent components, therefore, when an alloy is formed, it is considered as an independent component. For example, steel is an alloy, one component of which is iron, and the other is the chemical compound Fe3C (cementite).
Chemical compounds have very high hardness and good electrical resistance. Sometimes their hardness is 10 times higher than the hardness of pure components. For example, iron and carbon form the chemical compound Fe3C, the hardness of which is 10 times higher than the hardness of iron. Chemical compounds of tungsten and titanium with carbon (carbides), characterized by very high hardness, are used to make cutting tools. Unlike solid solutions, chemical compounds are characterized by high fragility and are unsuitable for pressure treatment.
Mechanical mixture. Individual components in the solid state do not dissolve in each other (to form a solid solution) and do not enter into a chemical reaction with each other (to form a chemical compound). During crystallization, each of these components creates its own crystal lattice, unique to it alone. Mixing with each other at a constant temperature and a certain percentage, they form a mechanical mixture, in which individual components are visible when examined under a microscope. Thus, a mechanical mixture of Pb - Sb is formed at a temperature of 246 ° C and the ratio of components: Pb -87%, Sb - 13%.
Mechanical mixtures have good casting properties. This especially applies to eutectic alloys, which have greater fluidity and a lower melting point than their constituent components.
Knowledge of the structure of alloys facilitates their selection in the manufacture of machine parts and the development of technological processes. When studying the processes occurring in metals and alloys when their temperature and composition change, concepts such as component, system, and phase are used.
, a system is a collection of substances or bodies between which energy and mass can be exchanged without hindrance. The system can be a chemical element (sulfur, aluminum, hydrogen), a chemical compound (Fe3C, water, table salt), an alloy of two or more metals (copper-nickel, tin - lead - antimony), an aqueous solution (sugar in water) , a mixture of gases (air consisting of nitrogen, oxygen, carbon dioxide and five inert gases).
A phase is a part of a system that has a homogeneous structure and is separated from other parts by an interface. A phase can include any number of components that make up the system. The system may contain one or more phases. Single-component systems “sulfur”, “aluminium”, “table salt” at room temperature have one solid phase, the “water” system under the same conditions contains one liquid phase, and the “hydrogen” system - one gaseous phase. At temperatures below zero, the “water” system is also single-phase, having one solid phase - ice. At zero degrees, this system is two-phase, since at this temperature liquid (water) and solid (ice) phases coexist.
The two-component system “sugar solution in water” is single-phase, that is, it has one liquid phase if the solution is unsaturated. The same system will be two-phase with a saturated solution containing undissolved sugar crystals, which are the second (solid) phase. The liquid phase in this case will contain two components (water and sugar), and the solid phase only one (sugar).
Industrial production
Iron-carbon alloys are produced by metallurgical plants from various components. The basis is iron with carbon. Stages of the production process:
- Preparation of consumable raw materials (ore). It is sorted by piece size and chemical composition. Low-grade ores are enriched with the required components. Waste rocks are removed.
- Fuel preparation. Coke coal undergoes a screening procedure. It is needed to remove foreign impurities from the fuel, which can lead to heat losses during ore smelting.
- Flux preparation. Substances that will be used for the production of cast iron are crushed. At the same time, fines are sifted out and foreign impurities are removed.
- Loading consumables and ore into the blast furnace. First, it is filled with coke, ore is laid out on top, and another layer of coke is poured on top of it. Heated air is blown inside to maintain the melting temperature of the metal. When coke burns, a large amount of carbon dioxide is released, which passes through the coke residues, forming the compound CO. During the reduction process, the iron gains hardness. Gradually the carbon begins to dissolve. Liquid cast iron is fed to special ladles, from which it is poured into molds.
Large blast furnaces are used to produce cast iron. Their height can reach 30 m, and their internal diameter is 12 m.
Blast furnace (Photo: Instagram / viktormacha)
Chemical compounds
The main structures that make up iron-carbon alloys:
Ferrite is a solid solution of carbon in α-Fe. At 723°C the maximum carbon content is 0.02%.It will not corrode if there are any impurities.
Cementite is a compound containing iron carbon fe3c-6.67% carbon carbide. The eutectic is an integral part of the mixture and an independent structural component. Due to the substitution of atoms of other metals, a solid solution can be formed, which is unstable and decomposes during heat treatment. Cementite is very hard (HB 800) and brittle.
Austenite is a solid solution of carbon in γ-Fe. Carbon atoms are introduced into the crystal lattice, and the degree of saturation can vary depending on temperature and impurities. It is stable only at high temperatures, and stable even at normal low temperatures - impurities of Mn, Cr. Austenitic hardness HB 170… 220.
Microstructure:
- a-hypereutectoid steel-ferrite (light area) and pearlite (dark area) at 500X magnification, b-eutectoid steel-perlite (1000'), c-eutectoid steel-interlocking pearlite and cementite (200').)
- The solubility of carbon in ferrite decreases from 727% at 0.02°C to 0.005% at room temperature.
Properties
Characteristics of iron-carbon alloys:
- Density - up to 7.9 g/cm3.
- Melting point - up to 1520 °C.
- Specific heat capacity - 462 J/(kg °C) at an ambient temperature of no more than 20 °C.
- Specific heat of fusion - 84 kJ/kg.
- Thermal conductivity coefficient is 30 W/(m K) at a temperature of 100 °C.
- The coefficient of linear thermal expansion is 11.5·10-6 1/°C.
Iron-carbon alloys are produced by industrial enterprises. These include different types of steel and cast iron. They are used in various industries.
Classification
Steel is classified according to different criteria. By chemical composition:
- High carbon - more than 0.65% C.
- Medium carbon - from 0.25% to 0.65% C.
- Low carbon - less than 0.25% C.
By structure:
- hypereutectoid;
- eutectoid;
- hypoeutectoid;
- ledeburite.
By purpose:
- Instrumental. Used in the manufacture of working parts and accessories for electrical tools and industrial equipment.
- Structural. They are used in the manufacture of metal structures, parts of industrial equipment, and machines.
Steel in rolls (Photo: Instagram / mmz_sim)
Iron-carbon alloys
Iron-carbon alloys may contain the following structural components:
- Ferrite (F) is a solid solution containing carbon and other elements in iron. It has a body-centered cubic lattice. The solubility of carbon in ferrite is very low, up to 0.005% at room temperature. At 727°C, the highest solubility of 0.02% ferrite is very ductile, soft and workable by applying pressure under cold conditions.
Austenite (a) is a solid solution of carbon and other G-iron elements. It is present only at high temperatures. the maximum solubility of carbon in g-iron is 1147% at 2.14°C and 727°C at 0.8%. This temperature is the lower limit for the presence of austenite in the iron-carbon alloy. Austenite is very ductile, but harder than ferrite.
Basic structures
An alloy of iron and carbon is the basis of steel and cast iron, called iron alloys, and these are the most important structural materials in technology.
The structure and properties of the alloy largely depend on the characteristics of the main components and additive elements, as well as the nature of their interaction.
Pure iron is a silvery-white metal, refractory. The melting point of iron is 1539°C. iron has 2 polymorphs a and G.
- At temperatures below 910°C, iron has a body-centered cubic lattice. This change is called A-iron. a-iron is a temperature up to 768°C (Curie point) magnetically.
When the iron is heated, the 910°C body-centered cubic lattice turns into a side-centered cubic lattice, and the A-iron turns into a G-iron. g-iron is present at a temperature of 910-1392°C
Carbon is a non-metallic element. The melting point of carbon is 3500°C. Carbon in nature exists in 2 polymorphic modifications: Diamond and graphite.
The diamond form is not found in the alloy.
In a carbon-iron alloy, the free carbon is in the form of graphite. The crystal structure of graphite is layered. Its strength and ductility are very low.
Carbon can dissolve in iron in a liquid or solid state, forming chemical compounds - cementite can be in free form in the form of graphite.
Nodal critical points of the iron-carbon system state diagram
On the iron-carbon diagram there are a number of points called critical points. Each point carries information about temperature, carbon content and a description of what exactly is happening in that place.
There are 14 of these critical points in total.
For example, A, says that at a temperature of 1539 ° C and at zero carbon content, pure iron melts. D indicates that at a temperature of 1260 Fe3c can melt.
The points are located at the intersection of lines placed on the diagram.