Hypereutectic cast iron
| State diagram of Fe - C. Secondary transformations into high-carbon compounds. |
In hypereutectic cast irons, the transformations discussed above occur, since primary cementite does not have transformations.
| Eutectic colonies of white zazvtek. tic cast iron. X 100. |
In hypereutectic cast irons, plates of primary cementite play the role of a lining on which eutectic crystallization begins.
When hypereutectic cast irons solidify, the eutectic transformation is preceded by the precipitation of primary cementite.
The cooling curve of hypereutectic cast iron containing 5% C (Ks) is shown in Fig.
Transformations in hypereutectic cast iron containing more than 4-3% C begin at liquidus temperatures with the release of cementite crystals. The carbon concentration in the liquid decreases with decreasing temperature along the CD line. The alloy hardens to form ledeburite. Hypereutectic cast irons do not have free carbon deposits in the form of graphite, since all carbon is bound in pearlite and cementite, and are characterized by high hardness.
To test this assumption, hypereutectic cast iron containing 3 7% C, 2 5% S, 0 003% 5ISKH was smelted in a magnesite crucible in an MVP-ZM induction furnace; after overheating to 1500 C, it was modified with cerium metal at 1460 C. The melt, cooled to 1180 C, was poured into water. Microanalysis of 0 4 - 6 mm pellets revealed spherical inclusions of excess graphite, surrounded by thin ledeburite, into which the liquid phase turned during quenching.
The formation of primary graphite in hypereutectic cast iron or graphite during eutectic transformation, as well as the formation of graphite due to the decomposition of primary or eutectic cementite, is called the first stage of graphitization.
The line of eutectic compositions SS delimits the areas of hypoeutectic and hypereutectic cast irons.
Similar transformations of eutectic austenite occur in hypereutectic cast irons; the structure of such cast irons below 723e C consists of primary cementite and ledeburite.
Similar transformations of eutectic austenite occur in hypereutectic cast irons; the structure of such cast irons below 723 C consists of primary cementite and ledeburite.
In zone VII of the diagram (Fig. 7), the structure of hypereutectic cast irons consists of ledeburite and primary cementite. At a temperature corresponding to the PSK line, austenite decomposes, forming pearlite. As mentioned above, under the influence of certain factors (slow solidification rate, content of additional components, mainly silicon), graphite can be formed instead of cementite. Cast irons without graphite (with ledeburite) are called white, with ledeburite and graphite - half-cast, and without ledeburite (with graphite) - gray.
In zone VII of the diagram (see Fig. 6 6), the structure of hypereutectic cast irons consists of ledeburite and primary cementite. At a temperature corresponding to the PSK line, austenite decomposes, forming pearlite. As mentioned above, under the influence of certain factors (slow solidification rate, content of additional components, mainly silicon), graphite can be formed instead of cementite. Cast irons without graphite (with ledeburite) are called white, with ledeburite and graphite - half-cast, and without ledeburite (with graphite) - gray.
| Reverse segregation of silicon in gray cast iron with 4 0% C and 1 95% Si. Etching with sodium picrate, X50. |
Components in the iron-carbon system
The components of iron-carbon alloys are iron, carbon and cementite:
Iron
Iron is a d-transition metal with a silvery-light color. Melting point – 1539° C. Specific gravity is 7.86 g/cm3. The most significant feature of iron is its polymorphism. In the solid state, iron can be found in two modifications - α and γ. Polymorphic transformations occur at temperatures of 911° C and 1392° C. At temperatures below 911° C and above 1392° C, Feα (or α-Fe) exists with a body-centered cubic lattice. In the temperature range 911…1392° C, Feγ (or γ-Fe) with a face-centered cubic lattice is stable. During the transformation α→γ, a decrease in volume is observed, since the γ-Fe lattice has a more dense packing of atoms than the α-Fe lattice. Upon cooling during the transformation γ→α, an increase in volume is observed. In the temperature range 1392…1539° C, high-temperature Feα is called Feδ. The high-temperature modification of Feα does not represent a new allotropic form.
At temperatures below 768° C, iron is ferromagnetic, and above it is paramagnetic. Point 768° C, corresponding to the magnetic transformation, i.e. The transition from a ferromagnetic state to a paramagnetic state is called the Curie point. The Feγ modification is paramagnetic.
Iron of technical purity has low hardness (80 HB) and strength (tensile strength – σв=250 MPa, yield strength – σт=120 MPa) and high plasticity characteristics (relative elongation – δ=50%, and relative contraction – ψ=80% ). Properties may vary within certain limits depending on the grain size. Iron is characterized by a high modulus of elasticity, the presence of which is also manifested in alloys based on it, providing high rigidity of parts made from these alloys.
Iron forms solutions with many elements: with metals - substitution solutions, with carbon, nitrogen and hydrogen - interstitial solutions.
Carbon
Carbon is a non-metal. It has a polymorphic transformation, depending on the conditions of formation, it exists in the form of graphite with a hexagonal crystal lattice (melting point - 3500 ° C, density - 2.5 g / cm3) or in the form of diamond with a complex cubic lattice with a coordination number of four (melting point – 5000° C).
In iron-carbon alloys, carbon is in a state of solid solution with iron and in the form of a chemical compound - cementite (Fe3C), and also in a free state in the form of graphite (in gray cast iron).
Cementite
Cementite (Fe3C) is a chemical compound of iron and carbon (iron carbide), contains 6.67% carbon. More precise studies have shown that cementite can have a variable carbon concentration. However, in the future, when analyzing the phase diagram, we will make the assumption that Fe3C has a constant composition. The crystal lattice of cementite is rhombic, specific gravity 7.82 g/cm3 (very close to the specific gravity of iron). At high temperatures, cementite dissociates, so its melting point is unclear and is approximately 1260° C. It does not experience allotropic transformations. The crystal lattice of cementite consists of a series of octahedra, the axes of which are inclined to each other. At low temperatures, cementite is weakly ferromagnetic, losing its magnetic properties at a temperature of about 210° C. Cementite has high hardness (more than 800 HB, easily scratches glass), but extremely low, almost zero, ductility.
Cementite is capable of forming substitutional solid solutions. Carbon atoms can be replaced by non-metal atoms: for example, nitrogen; iron atoms - metals: manganese, chromium, tungsten, etc. Such a solid solution based on a cementite lattice is called alloyed cementite.
If graphite is a stable phase, then cementite is a metastable phase. Cementite is an unstable compound and, under certain conditions, decomposes to form free carbon in the form of graphite
This process is of great practical importance in the structure formation of cast irons.
Structure and properties
Cementite
The main phase that initiates the nucleation of ledeburite is cementite. A flat austenite dendrite grows on a cementite plate nucleated in the eutectic fluid. This is followed by a relatively rapid pairwise growth of mutually germinated crystals of both phases. Each of the phases within one ledeburite colony is continuous, that is, it belongs to one crystal.
Depending on the temperature, the phase composition of ledeburite can be different. Thus, in the temperature range from 1147 °C to 727 °C, ledeburite consists of austenite and cementite, and at temperatures below 727 °C - of ferrite and cementite.
Ledeburite has high hardness and brittleness.
Normal blood iron level
The level of iron in the blood depends on the gender and age of a person. Due to the constitutional characteristics of the body, metabolic rate, and lower muscle mass, women have a lower need for iron than men.
The indicator fluctuates:
- in girls from sixteen to eighteen years old, the microelement norm is 5.8-18 µmol/l;
- during the reproductive period it increases to 11.5-30.4;
- in the period after 45 to 65 years 12-31;
- after 65 years of age the rates decrease slightly.
Iron levels in pregnant women, depending on the period, should fluctuate between 8.9-30.3 µmol/l.
For men:
- in adolescence, the norm is 5-25 µmol/l;
- from 19 to 45 years old it increases to 11.5-30.5;
- 45-65 years 12-32 µmol/l.
In men over sixty-five years of age, the norm decreases to 12-29.7 µmol/l.
In newborns in the first month of life, the iron norm is 5-20 µmol/l, in babies up to one year it is 5-21, up to five years old 5-18, up to 11 – 5.5-11.5.
Even knowing the level of iron in the blood, it is impossible to understand the tests on your own, since laboratory data is still taken into account in the system.
Martensite structure
Fe-fe3c diagram components, phases, lines and points
The main difference that leads to a change in the physical and mechanical characteristics of steel is a change in the internal structure. It is called martensitic structure. In this case, the crystal lattice undergoes the following changes. Under the influence of external factors, the direction of movement of atoms changes compared to their standard, ordered movement within the established lattice. Interatomic distances increase, which leads to deformation by approximately 10% relative to normal sizes. The magnitude of the changes does not lead to a transition through the energy barrier of interatomic bonds. This crystalline effect leads to the formation of a specific form of mutual bonds. It has a so-called needle-like character.
Changes in the structure of steel occur during the heating process. An increase in temperature causes a diffusion redistribution of carbon atoms within the crystal lattice. This process causes the formation of several metal phases.
- When the carbon content increases to 6.7%, a material called cementite appears. It has a diamond-shaped grille.
- At low carbon content (no more than 0.02%), ferrite is formed. Its lattice takes on a body-centered shape.
- Austenite. The structure of iron-carbon alloys, which are a mixture of carbon in an amount of about 2% of various alloying additives. The crystal lattice of this material has the shape of a cube with strictly centered edges. A distinctive feature of austenite is its high density compared to other steel structures. It is formed at heating temperatures from 910 to 1401 °C and remains stable up to a temperature of 723 °C. With further cooling it turns into other more stable structures. With the addition of nickel, manganese or chromium, austenite retains its structure down to room temperature. Steels with an austenitic structure include almost all chromium-nickel steels.
- Perlite is a mechanical mixture of cementite and ferrite. In this mixture the presence of carbon is 0.8%. It is formed from austenite during the cooling process. It is a eutectoid and can have a plastic or granular structure. Its physical and especially mechanical properties depend on this state.
- When the carbon content is increased to 4.3%, a material called ledeburite is formed from the mixture of austenite and cementite. Its formation occurs at a melt temperature of 1147 °C.
- Martensite is a supersaturated solution of iron and carbon. It is usually obtained by hardening austenite. As a result of temperature exposure, the martensitic material acquires a tetragonal lattice from a cubic one, which gives it a hardness of up to 1000 HV.
As a result of processing, the resulting martensitic steel acquires a needle-like structure, which forms higher strength characteristics and becomes more resistant to corrosion.
Iron-carbon alloys
Iron-carbon alloys may contain the following structural components:
Ferrite (F) is a solid solution containing carbon and other elements in iron. It has a body-centered cubic lattice. The solubility of carbon in ferrite is very low, up to 0.005% at room temperature. At 727°C, the highest solubility of 0.02% ferrite is very ductile, soft and workable by applying pressure under cold conditions.
Austenite (a) is a solid solution of carbon and other G-iron elements. It is present only at high temperatures. the maximum solubility of carbon in g-iron is 1147% at 2.14°C and 727°C at 0.8%. This temperature is the lower limit for the presence of austenite in the iron-carbon alloy. Austenite is very ductile, but harder than ferrite.
Structure and properties
Welding of austenitic steels
The main phase that initiates the nucleation of ledeburite is cementite. A flat austenite dendrite grows on a cementite plate nucleated in the eutectic fluid. This is followed by a relatively rapid pairwise growth of mutually germinated crystals of both phases. Each of the phases within one ledeburite colony is continuous, that is, it belongs to one crystal.
Depending on the temperature, the phase composition of ledeburite can be different. Thus, in the temperature range from 1147 °C to 727 °C, ledeburite consists of austenite and cementite, and at temperatures below 727 °C - of ferrite and cementite.
Ledeburite has high hardness and brittleness.
Compound
Pearlite is a mechanical mixture of ferrite and cementite, formed during the eutectoid decomposition of slowly cooled austenite. The carbon concentration in perlite is 0.80%.
The hardness of pearlite is HB 180÷220. Steel containing 0.80% C has a pure pearlite structure.
Redebrite is a mechanical mixture of austenite and cementite formed during the crystallization of liquid alloys containing 4.3% C. This transformation also involves the austenite that is part of the redebrite, since austenite undergoes pearlite at a temperature of 723°C. therefore, at a temperature of 723° or below, red Britt is not a mixture of austenite and cementite, but a mixture of pearlite and cementite.
The main components of perlite: silicon dioxide SiO2 (65-75%), aluminum oxide AI2O3 (10-16%), potassium oxide K2O (up to 5%), sodium oxide Na2O (up to 4%), iron oxide Fe2O3 (up to 3 fractions) %, magnesium oxide (up to 1% of fractionation), calcium oxide CaO (up to 2%), water (H2O) (2 ~ 6). Other impurities may be present.
Perlite is characterized by a small concentric shell structure (pearlitic structure), resulting in a round core (Pearl) resembling a pearl with a characteristic luster.
Porosity 8-40%. Perlite can have a variety of shades: black, green, red-brown, brown, white. Types of perlite: Obsidian (contains obsidian impurities), spherulites (contains feldspar impurities), resinous stone (uniform composition), glassy and so on. Perlite, in the form of a massive, striped, angular rectification rock, pumice, is distinguished by its texture.
Expanded perlite is a loose, porous, loose, lightweight and durable material.
Fire resistance: operating temperature - minus 200-900°C. Can absorb up to 400% of liquid of its own weight: thermal insulation and sound insulation, maximum absorbency. It is biologically resistant. It is not subject to decomposition and rotting under the influence of microorganisms and is not a favorable habitat for insects and rodents.
Chemically inert: neutral to alkaline and weak acids. Perlite is an environmentally friendly, non-toxic and sterile material and does not contain heavy metals.
Perlite can be used naturally (in construction), but expanded perlite is more often used. The use of expanded perlite in construction makes it possible to increase the thermal insulation and sound insulation of structures under construction and fire protection characteristics, while significantly reducing the weight and volume of structures.
Degree - eutecticity - cast iron
The degree of eutecticity of cast iron is determined by the proportion of eutectic in its structure. In the case of pure iron-carbon alloys, it is calculated according to the lever rule on the EC (or E C) eutectic transformation (Fig.
The degree of eutecticity of cast iron is determined by the proportion of eutectic in its structure.
| Structural diagram of N. G. Girsho. |
The straight lines Ca in the diagram show the degree of eutecticity of cast iron, taking into account the carbon equivalent. If this value is 4 25, then the cast iron is eutectic; if it is less than 4 25, then the cast iron is hypoeutectic.
The diagrams reflect the influence of silicon on the degree of eutecticity of cast iron: as its content increases, the liquidus of TB decreases and the two-phase region F - - A narrows.
| Girshovich-Ioffe structural diagram (unmodified cast iron. Structure of the metal base. I - pearlite. C - cementite. F - normal ferrite. F - abnormal ferrite. Graphite structure. / and / / - lamellar non-oriented and interdendritic. / / / - point. |
It was also shown above that the content and structure of the graphite phase in cast iron are related to the degree of eutecticity of cast iron, which is confirmed by analysis of the Girshovich-Ioffe diagrams. Therefore, the connection diagram was also based on a family of lines - isoeutectics, which represent the geometric locus of figurative points of cast iron with the same degree of eutecticity and dividing the connections into proportional segments.
The Collot diagram and the Cola nomogram reflect the dependence of tensile strength, hardness and elastic modulus on the degree of eutecticity of cast iron.
| Gas content in cast iron depending on the degree of eutecticity 5E. |
Of the total amount of gases in cast iron, the hydrogen content is 50 - 65%, it decreases with increasing degree of eutecticity of cast iron. The amount of hydrogen released during storage is about 80% of its total content.
A comparison of the kinetic diagrams of crystallization of chromium cast irons near the eutectic composition (Fig. 64) shows that an increase in chromium content does not have a noticeable effect on the relative position of the line of appearance of austenite BZ; therefore, chromium has little effect on the degree of eutecticity of cast iron. The temperature range for the release of graphite decreases and at concentrations of 1–92% Cr, graphite does not separate from the melt. The shift of the OF line to the right indicates that with increasing chromium content, the separation of graphite becomes more difficult. It is appropriate to compare with this the influence of manganese (see Fig. 58), an increase in the content of which in cast iron does not lead to an extension of the incubation period for the appearance of graphite in the liquid.
When hypoeutectic cast irons solidify, excess austenite first crystallizes. As in gray pre-eutectic cast iron, it grows in the form of three-dimensional dendrites. Their number, size and branching are determined by the degree of eutecticity of the cast iron and cooling conditions. Then, simultaneous crystallization of austenite and cementite occurs during the eutectic decomposition of the liquid solution.
The formation of nodular graphite in high-silicon cast irons occurs only under conditions that provide significant supercooling of the melt. In the practice of casting production, the formation of highly branched, including interdendritic graphite during the accelerated solidification of cast iron with a high silicon content is of great importance. In the case of slow crystallization of cast iron, on the contrary, an increase in the silicon content leads to the formation of coarser graphite. This is a consequence of the increase in the degree of eutecticity of cast iron when silicon is added to it and is found primarily in low-carbon cast irons.
Application of perlite
The white color of perlite makes it difficult to diagnose soil pests (root-eating insects, mealybugs, fungal larvae). Perlite pH is neutral. When a plant is grown in pure perlite and irrigated with hard water, the pH of the substrate can shift to the alkaline side, which inhibits plant growth and prevents the use of nutrients.
Because it has a positive charge, it cannot retain the positive ion of the fertilizer and does not participate in the ion exchange process.
Vermiculite, brick chips, fine expanded clay, polystyrene chips, sand (the last 2 components give the substrate porosity and looseness, but do not retain water).
Presence in iron-carbon alloys
Cast iron
The ledeburite mixture occurs for pure iron-carbon alloys in the range of carbon concentrations from 2.14% to 6.67%, which corresponds to cast iron. The mechanism of ledeburite formation in hypoeutectic (to the left of the eutectic point corresponding to 4.3 carbon on the iron-carbon diagram), eutectic and hypereutectic (to the right of the eutectic point) cast irons differs.
in hypoeutectic cast irons
When the liquid phase of the hypoeutectic cast iron composition is cooled, austenite begins to crystallize first, as a result of which the composition of the liquid phase begins to shift towards increasing carbon concentration (due to the lower solubility of carbon in austenite). Upon reaching the eutectic point (4.3% carbon, 1147 °C), crystallization of the eutectic, ledeburite, begins. During further cooling of cast iron in the temperature range from 1147 °C to 727 °C, austenite is depleted of carbon and secondary cementite is released. Secondary cementite, released along the boundaries of austenite grains, merges with ledeburite cementite and is therefore practically invisible under a microscope. With slight supercooling below 727 °C, austenite transforms into pearlite by a eutectoid reaction (divided into ferrite and cementite). Thus, in hypoeutectic white cast irons, at room temperature, ledeburite, as a structural component, is present along with pearlite and secondary cementite.
in eutectic cast iron
When the liquid phase of the eutectic point composition is cooled to a temperature of 1147 °C, simultaneous crystallization of a mixture of austenite and cementite - ledeburite begins. Subsequently, the austenite decomposes into a ferrite-cementite mixture (pearlite).
in hypereutectic cast irons
In hypereutectic white cast irons, primary cementite crystallizes from the liquid in the form of flat needles, then ledeburite is formed. At room temperature, eutectic white cast iron contains two structural components: primary cementite and ledeburite.
Ledeburite can form in steels if, firstly, their carbon content is high enough (over 0.7% (~1.3%-1.5%), which corresponds to tool steels), and, secondly, at high content of carbide-forming alloying elements (Cr, W, Ti, Mo, etc.). The introduction of these alloying elements, in large quantities, reduces the solubility of carbon in austenite and pearlite, which, in certain cases, leads to the possibility of eutectic precipitation at relatively low carbon contents. Such steels (for example, high-speed steels) are called ledeburite.
Metastable iron-cementite diagram
The metastable diagram is also called “cementite”. The difference from the stable (“graphite”) diagram occurs when carbon is released - in the form of graphite or cementite.
As shown in the double diagram, the lattices of the allotropic forms of iron (δ, γ and α or delta, gamma and alpha) serve as the "solvent" for the formation of respectively solid δ-, γ- and α-solutions of carbon in iron.
When iron with a very low carbon content crystallizes, crystals of the solid delta solution are released along the liquidus line AB and the solidus line AN. The solid delta solution has a body-centered cubic (bcc) lattice - exactly the same as the solid alpha solution. At a maximum temperature of the delta solid solution of 1490 °C, the carbon content is 0.1% (point H). At a temperature of 1490 °C, a peritectic reaction occurs between a supersaturated solid δ-solution and a liquid phase containing 0.5% carbon (point B). As a result, a solid γ-solution of carbon in iron is formed. It contains 0.18% carbon (point I).
If the carbon content is higher than 0.5%, then the solid γ-solution crystallizes directly from the liquid phase (along the liquidus line BC and the solidus line IE). At a temperature of 1130 ˚C, the limited solubility of carbon in γ-iron is about 2.0% (point E). A decrease in temperature below 1130 °C leads to a decrease in the solubility of carbon in γ-iron along the ES line. At a temperature of 723 °C, the solubility of carbon is 0.8% (point S). The ES line corresponds to the release of iron carbide (cementite) from γ-iron.
As the carbon content increases, the temperature at which the gamma lattice transforms into the alpha lattice decreases, and this transformation occurs in the temperature range corresponding to the GS and GP lines.
The alpha phase precipitation line GS intersects with the iron carbide precipitation line ES at point S. Point S is a eutectic point with the following coordinates: 723 °C in temperature and 0.80% in carbon content. At this point, a solid alpha solution (ferrite) and iron carbide (cementite) are simultaneously released from a gamma solution of eutectoid composition.
The lattice of an alpha solid solution is identical to the lattice of a delta solid solution. At the eutectoid temperature of 723 °C, the alpha solid solution contains 0.02% carbon (point P). Further cooling leads to a decrease in the solubility of carbon in alpha iron and at room temperature it is only a small fraction of a percent (point D).
When the carbon content is between 2.0 and 4.3%, crystallization begins with the release of gamma solution along the BC line. An increase in carbon content above 4.3% leads to the precipitation of iron carbide along the CD line.
The precipitation of an additional primary phase in alloys with a carbon content above 2.0% is accompanied by eutectic crystallization of gamma solution and carbon carbide at point C, the coordinates of which are 1130 °C and 4.3% carbon.
The MO line is associated with the magnetic transformation, that is, the transition from the ferromagnetic to the paramagnetic state of iron.
Structure and properties
The main phase that initiates the nucleation of ledeburite is cementite. A flat austenite dendrite grows on a cementite plate nucleated in the eutectic fluid. This is followed by a relatively rapid pairwise growth of mutually germinated crystals of both phases. Each of the phases within one ledeburite colony is continuous, that is, it belongs to one crystal.
Depending on the temperature, the phase composition of ledeburite can be different. Thus, in the temperature range from 1147 °C to 727 °C, ledeburite consists of austenite and cementite, and at temperatures below 727 °C - of ferrite and cementite.
Ledeburite has high hardness and brittleness.
Iron - Carbon phase diagram.
Pure iron
Cementite is a carbonized compound of iron and carbon (fe3c). Cementite contains 6.67% carbon. The melting point of cementite is about 1600°C. It has a complex crystal lattice. Iron is the hardest and most brittle component of a carbon alloy. Cementite is unstable; under certain conditions, the reaction Fe3C> 3Fe + C causes the formation and decomposition of free carbon in the form of graphite.
The more cementite in an iron-carbon alloy, the higher the hardness.
Graphite is an allotrope of carbon. Graphite is soft and its strength is very low. Cast iron and graphitized steel are included in the composition in the form of inclusions of various shapes.
Perlite (P) is a mechanical mixture of ferrite and cementite containing 0.8% carbon. It is formed during recrystallization (collapse) of austenite at a temperature of 727°C. this decomposition is called eutectoid, and pearlite-eutectoid. Pearlite has high strength and hardness, which increases the mechanical properties of the alloy.
Redebrite is a mechanical mixture of austenite and cementite containing 4.3% carbon. It is formed as a result of eutectic transformation at a temperature of 1147 ° C. At a temperature of 727 ° C, austenite turns into pearlite, and after cooling, the red briquette turns into a mixture of pearlite and cementite. Redebrite has high hardness and excellent brittleness. Everything white is part of cast iron.
Hypoeutectic cast iron
Graphite formations (10 - 100 A) that appear above the liquidus line in hypoeutectic cast irons have a developed surface, and the properties of such a system (liquid dispersed formations) depend on the properties and sizes of the interfaces included in it. Graphite pinacoids are stable formations. Exposure at 1700 C does not completely eliminate microinhomogeneity of the melt. Thus, the microheterogeneity of cast iron melts has a hereditary origin associated with incomplete dissolution of carbon during the smelting process. Based on experimental data, it can be assumed that dispersed graphite precipitation begins above the liquidus temperature.
Titanium lowers the temperature of the eutectic transformation and promotes supercooling of cast iron; at a content of up to 0 5% in hypoeutectic cast iron, it promotes graphitization and the release of graphite in the form of small plates. Titanium is a good deoxidizer and promotes uniform distribution of graphite in cast iron. Titanium neutralizes the effect of chromium in cast iron, being a modifier, as a result of which there is no need to increase the silicon content. Titanium helps improve the mechanical properties, especially the strength of high-carbon cast irons. At a content of 0 18 - 0 20% titanium and carbon form carbides and prevent graphitization. Titanium is used as a modifier in the production of malleable cast iron, but for castings made of high-strength cast iron, Ti is an undesirable admixture, since it prevents the formation of nodular graphite.
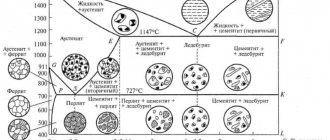
Depending on the carbon concentration in the alloy, cast irons are divided into hypoeutectic, eutectic, and hypereutectic: hypoeutectic cast irons contain 2 14 - 4 3% C and have a perlite - b cementite structure; eutectic cast irons contain 4-3% C and consist of ledeburite; hypereutectic cast iron contains more than 4-3% C and has a ledeburite-cementite structure.
Let us consider, in accordance with the iron-carbon phase diagram, the phase transformations that occur during cooling from the liquid state in hypoeutectic cast iron containing 3% C.
The same decomposed austenite is also observed in excess (dark) areas contained in greater or lesser quantities in hypoeutectic cast irons (Fig.
The fluidity of cast iron characterizes the filling of the casting mold and depends mainly on the chemical composition and temperature of the pouring. C, Si, P and Cu increase the fluidity of hypoeutectic cast iron, and S and Cr reduce it; the influence of Mn and Ni on fluidity is insignificant.
Point C (4-3% carbon) is a eutectic point and divides alloys containing from 2 to 6-67% carbon (cast irons) into two groups: alloys containing less than 4-3% C are hypoeutectic cast irons, and alloys containing more 4 3% C, - hypereutectic cast iron. It should be emphasized that in mechanical engineering, hypoeutectic and eutectic cast irons are of practical importance, but hypereutectic cast irons are not used.
| Effect of carbon on the hardness of chromium cast iron with silicon content, %. |
The eutectic composition of cast irons with 30 - 35% C is - 2 5% C. Hypoeutectic cast iron has the structure of a solid solution of chromium ferrite and eutectic carbides, the amount of which depends on the carbon content.
The solid phase in the region lying between the EGF and PSK lines with a carbon content of more than 2-14%, corresponding to white cast iron, has a different composition. Hypoeutectic cast irons (2 14 - 4 3% carbon) consist of austenite and ledeburite, eutectic (4 3%) of ledeburite and hypereutectic (4 3 - 6 67%) of cementite and ledeburite. Moreover, unlike steels, the melting point of cast iron (EGF line) is constant and does not depend on the carbon content in them.
Based on their structural properties, cast irons are divided into hypoeutectic and hypereutectic with respect to the eutectic composition of 4 3% C. Hypoeutectic cast irons have a pearlite-ledeburite structure, and hypereutectic ones have a cementite-ledeburite structure.
All cast irons contain austenite. In hypoeutectic cast irons there is free austenite (see alloy / - /, fig.
Finally, in hypoeutectic cast irons, primary austenite precipitates change their concentration upon cooling from point 3 to point 4 (alloy / CJ from 2 to 0 8% C, and pearlite transformation occurs at point 4. The structure of such hypoeutectic cast iron consists of pearlite, ledeburite and secondary cementite The structure of hypoeutectic cast iron is shown in Fig.
Finally, in hypoeutectic cast irons, the primary precipitates of austenite change their concentration upon cooling from point 3 to point 4 (KJ alloy) from 2 14 to 0 8% C, and at point 4 pearlite transformation occurs. The structure of such hypoeutectic cast iron consists of pearlite, ledeburite and secondary cementite.
Phases in the iron-carbon system
In the iron-carbon system there are the following phases: liquid phase, ferrite, austenite, cementite, graphite.
Liquid phase
Liquid phase. In the liquid state, iron readily dissolves carbon in any proportions to form a homogeneous liquid phase.
Ferrite
Ferrite (F, α) is a solid solution of carbon interpolation in α-iron (from the Latin word ferrum - iron). There are low-temperature ferrite with a maximum carbon solubility of 0.02% at a temperature of 727 ° C (point P) and high-temperature δ-ferrite (in the temperature range 1392 ... 1539 ° C) with a maximum carbon solubility of 0.1% at a temperature of 1499 ° C (point J).
The properties of ferrite are close to those of iron. It is soft (hardness - 80 - 130 HB, tensile strength - σв = 300 MPa) and plastic (relative elongation - δ = 50%), magnetic up to 768 ° C.
Under a microscope, ferrite appears as light polyhedral grains. In steels it can exist in the form of a mesh (of varying thickness, depending on the carbon content), grains (low-carbon steels), plates or needles (Widmanstätt).
Austenite in steels
Austenite (A, γ) is a solid solution of carbon intercalation into γ-iron (named after the English scientist R. Austen). Carbon occupies a place in the center of a face-centered cubic cell. The limiting solubility of carbon in γ-iron is 2.14% at a temperature of 1147 ° C (point E). Austenite has a hardness of 180 HB, is plastic (relative elongation – δ=40...50%), and paramagnetic. When other elements are dissolved in austenite, the properties and temperature limits of existence may change. Under a microscope it looks like light polyhedral grains with twins.
Cementite - forms of existence
In iron-carbon alloys there are phases: primary cementite, secondary cementite, tertiary cementite. The chemical and physical properties of these phases are the same. The mechanical properties of alloys are influenced by differences in the size, quantity and location of these precipitates. Primary cementite is released from the liquid phase in the form of large lamellar crystals. Secondary cementite is released from austenite and is located in the form of a network around austenite grains (when cooled, around pearlite grains). Tertiary cementite is released from ferrite and is located in the form of small inclusions at the boundaries of ferrite grains.
Since carbon in alloys with iron occurs in the form of cementite and graphite, there are two phase diagrams that describe the conditions of phase equilibrium in the iron-cementite and iron-graphite systems. The first diagram (Fe - Fe3C) is called cementite (metastable), the second (Fe - C) is called graphite (stable). Both versions of the diagram are shown together in the same coordinate system: temperature - carbon content. The state diagram of the iron-carbon system was constructed based on the results of numerous studies conducted by scientists in a number of countries. A special place among them is occupied by the works of D.K. Chernova
He discovered the existence of critical points in steel, determined their dependence on carbon content, and laid the foundation for constructing a state diagram of iron-carbon alloys in its lower, most important part
The letter designation of nodal points in the diagram is generally accepted both in Russia and abroad.
Iron-carbon phase diagram
The phase diagrams present in all areas are visible in the figure. The meaning of all lines is indicated in the table.
Liquidus throughout the diagram runs along the lines AB, BC, CD; solidus - along the lines AN, HJ, JE, ECF. Alloys of iron and carbon are usually divided into steel and cast iron. The conventional limit for such division is 2.14% C (point E). Alloys containing less than 2.14% carbon are classified as steels, and more than 2.14% are classified as cast iron.
Temperatures at which phase and structural transformations occur in alloys of the iron-cementite system, i.e. critical points have symbols. They are designated by the letter A. Depending on whether the critical point is determined during heating or cooling, the index c (from the word chauffage - heating) is added to the letter A when heating and the index r (from the word refroidissement - cooling) when cooling, leaving a number characterizing this transformation.
Thus, for example, heating of hypoeutectoid steel above the corresponding point on the GS line is designated as heating above point AC3. When cooling this steel, the first transformation should be designated as Ar3, the second (on the RSK line) - as Ar1.
Structure and properties
The main phase that initiates the nucleation of ledeburite is cementite. A flat austenite dendrite grows on a cementite plate nucleated in the eutectic fluid. This is followed by a relatively rapid pairwise growth of mutually germinated crystals of both phases. Each of the phases within one ledeburite colony is continuous, that is, it belongs to one crystal.
Depending on the temperature, the phase composition of ledeburite can be different. Thus, in the temperature range from 1147 °C to 727 °C, ledeburite consists of austenite and cementite, and at temperatures below 727 °C - of ferrite and cementite.
Ledeburite has high hardness and brittleness.
Primary, secondary and tertiary cementite
According to the method and field of education, it is divided into three main types:
- primary;
- secondary;
- tertiary.
The formation of primary cementite is observed during the crystallization of hypereutectic cast iron. At this moment, elongated crystals form. They form primary carbide. Primary formation can manifest itself in hypoeutectic cast iron in the composition of ledeburite during the crystallization of the melt. Studies have shown that this mixture of iron and carbon is present not only in white cast iron. It can appear in gray cast iron after completion of the so-called graphitization operation.
The process of formation of the secondary type is observed mainly during cooling of austenite. This phenomenon is observed when the temperature drops below 1147 °C. At this temperature, the carbon concentration in austenite decreases. The released carbon atoms enter into new bonds, and cementite is formed, which is called secondary. With a further decrease in temperature to eutectoid, its formation continues. Even at room temperature it is found in perlite. Under these conditions it can be found in hypereutectoid steel. It forms at the boundaries of the granular structure.
The cooling process of ferrite forms what is called tertiary cementite. This species is quite difficult to record, and further monitoring of its formation is being carried out. This problem is associated with the appearance of tertiary cementite in small quantities. Studies of the formation of this fraction have shown that it takes on several forms: plates, veins or in the form of needles. All these elements are formed in ferrite grains. Tertiary formation is quite difficult to obtain because as the percentage of carbon increases, tertiary cementite combines with perlite. As the cooling rate increases, the carbon content is retained in the metal solution and the formation of the tertiary fraction stops. A clear sign of formation is the result of gradual aging of ferrite. In this case, the carbon concentration in the ferrite content changes.
From the above description the following conclusions can be drawn:
- the primary fraction is formed as a result of crystallization of the melt;
- secondary – as a result of sequential cooling of austenite;
- tertiary – after cooling the ferrite.
In different grades of steel and cast iron, primary cementite has a high variability of shape. These can be plates of regular strip shape or needle-shaped formations. During the annealing operation, it can take the form of rounded formations. As a result, it transforms into granular perlite.
Structure and properties
The main phase that initiates the nucleation of ledeburite is cementite. A flat austenite dendrite grows on a cementite plate nucleated in the eutectic fluid. This is followed by a relatively rapid pairwise growth of mutually germinated crystals of both phases. Each of the phases within one ledeburite colony is continuous, that is, it belongs to one crystal.
Depending on the temperature, the phase composition of ledeburite can be different. Thus, in the temperature range from 1147 °C to 727 °C, ledeburite consists of austenite and cementite, and at temperatures below 727 °C - of ferrite and cementite.
Ledeburite has high hardness and brittleness.
Free cementite
Free cementite (Fe3C), which is formed when there is insufficient silicon, too much manganese and sulfur.
Structurally free cementite is undesirable.
Structurally free cementite, X 500: a – before deformation, 6 – after deformation.
Decomposition of structurally free cementite is achieved by heating and holding the casting above a critical interval; The heating temperature and holding time depend on the composition of white cast iron in terms of carbon content (Fig.
The amount of structurally free cementite. Inclusions of structurally free cementite located along the boundaries of ferrite grains (Fig. Inclusions of structurally free cementite coagulated and located inside ferrite grains are less dangerous. The scale is built according to the increasing size of cementite inclusions and the development of its distribution in the form of a network or chain.
Decomposition of structurally free cementite is achieved by heating and holding the casting above a critical interval; The heating temperature and holding time depend on the composition of white cast iron in terms of carbon content (Fig.
Particles of structurally free cementite should be small, as evenly dispersed as possible (Fig. Small particles of cementite are obtained at low temperatures of winding hot-rolled strip into a roll, and large ones - at high temperatures, when they have time not only to separate from the solid solution in ferrite, but also to reach large sizes due to coagulation and growth.
The amount of structurally free cementite in steel is determined by scores based on reference samples of microstructures.
In addition to structurally free cementite, there is also tertiary cementite at the boundaries of ferrite grains. It is impossible to prevent its release during the final heat treatment, since for this purpose the sheets for deep drawing must be cooled slowly.
Structurally free cementite is not allowed. Eutectic graphite and ferrite are allowed in the form of separate small inclusions in an amount of no more than 5% of the section area for each inclusion. The casting fracture must have a uniform fine-grained structure with a matte tint.
SE), the free cementite present in it will completely dissolve in austenite and the structure will become homogeneous.
Hypoeutectoid steels do not contain structurally free cementite.
C) Cast irons with structurally free cementite are classified as white cast irons. Ferrite may appear in them as a result of annealing, but such cast iron is not ferritic.
Structurally free cementite is not allowed in low carbon steel. It is formed as a result of slow cooling after rolling or heat treatment and, located along the grain boundaries, sharply reduces the plastic properties. This causes large rejects during cold heading.