4.2
Average rating: 4.2
Total ratings received: 248.
4.2
Average rating: 4.2
Total ratings received: 248.
The physical properties of metals distinguish them from non-metals. All metals, except mercury, are solid crystalline substances that are reducing agents in redox reactions.
Table of hardness of metals according to the Mohs scale:
| Hardness | Metal |
| 0.2 | Cesium |
| 0.3 | Rubidium |
| 0.4 | Potassium |
| 0.5 | Sodium |
| 0.6 | Lithium |
| 1.2 | Indium |
| 1.2 | Thallium |
| 1.25 | Barium |
| 1.5 | Strontium |
| 1.5 | Gallium |
| 1.5 | Tin |
| 1.5 | Lead |
| 1.5 | Mercury |
| 1.75 | Calcium |
| 2.0 | Cadmium |
| 2.25 | Bismuth |
| 2.5 | Magnesium |
| 2.5 | Zinc |
| 2.5 | Lanthanum |
| 2.5 | Silver |
| 2.5 | Gold |
| 2.59 | Yttrium |
| 2.75 | Aluminum |
| 3.0 | Copper |
| 3.0 | Antimony |
| 3.0 | Thorium |
| 3.17 | Scandium |
| 3.5 | Platinum |
| 3.75 | Cobalt |
| 3.75 | Palladium |
| 3.75 | Zirconium |
| 4.0 | Iron |
| 4.0 | Nickel |
| 4.0 | Hafnium |
| 4.0 | Manganese |
| 4.5 | Vanadium |
| 4.5 | Molybdenum |
| 4.5 | Rhodium |
| 4.5 | Titanium |
| 4.75 | Niobium |
| 5.0 | Iridium |
| 5.0 | Ruthenium |
| 5.0 | Tantalum |
| 5.0 | Technetium |
| 5.0 | Chromium |
| 5.5 | Beryllium |
| 5.5 | Osmium |
| 5.5 | Rhenium |
| 6.0 | Tungsten |
| 6.0 | β-Uranium |
Chemical properties of metals
Metals easily give up electrons, i.e. they are reducing agents. Therefore, they react easily with oxidizing agents.
Questions
- Which atoms are oxidizing agents?
- What are the names of simple substances consisting of atoms that are capable of accepting electrons?
Thus, metals react with non-metals. In such reactions, nonmetals, when accepting electrons, usually acquire a LOWER oxidation state.
Let's look at an example. Let aluminum react with sulfur:
Question. Which of these chemical elements is only capable of donating electrons? How many electrons?
Aluminum is a metal that has 3 electrons on its outer level (group III!), so it donates 3 electrons:
As the aluminum atom gives up electrons, the sulfur atom accepts them.
Question. How many electrons can a sulfur atom accept before completing the outer level? Why?
The sulfur atom has 6 electrons in its outer level (group VI!), therefore, this atom receives 2 electrons:
Thus, the resulting compound has the composition:
As a result, we obtain the reaction equation:
Task 8.5. Using similar reasoning, compose reaction equations:
- calcium + chlorine (Cl2);
- magnesium + nitrogen (N2).
When composing reaction equations, remember that a metal atom gives up all its external electrons, and a non-metal atom accepts as many electrons as there are missing up to eight.
The names of compounds obtained in such reactions always contain the suffix ID :
The root word in the name comes from the Latin name for a non-metal (see lesson 2.4).
Metals react with acid solutions (see lesson 2.2). When drawing up equations for such reactions and when determining the possibility of such a reaction, one should use a series of voltages (activity series) of metals:
Metals in this series before hydrogen are capable of displacing hydrogen from acid solutions:
Task 8.6. Write down equations for possible reactions:
- magnesium + sulfuric acid;
- nickel + hydrochloric acid;
- mercury + hydrochloric acid.
All these metals in the resulting compounds are divalent.
The reaction of a metal with an acid is possible if it results in a soluble salt. For example, magnesium practically does not react with phosphoric acid, since its surface is quickly covered with a layer of insoluble phosphate:
Metals after hydrogen can react with some acids, but hydrogen is not released in these reactions:
Task 8.7. Which of the metals - Ba, Mg, Fe, Pb, Cu - can react with a solution of sulfuric acid? Why? Write down equations for possible reactions.
Metals react with water if they are more active than iron (iron can also react with water). In this case, very active metals ( Li – Al ) react with water under normal conditions or with slight heating according to the following scheme:
where x is the valency of the metal.
Task 8.8. Draw up reaction equations according to this scheme for K, Na, Ca. What other metals can react with water in this way?
The question arises: why does aluminum practically not react with water? Indeed, we boil water in an aluminum pan, and... nothing! The fact is that the surface of aluminum is protected by an oxide film (relatively Al2O3). If it is destroyed, a reaction of aluminum with water will begin, and quite active. It is useful to know that this film is destroyed by chlorine ions Cl–. And since aluminum ions are unsafe for health, you should follow the rule: you cannot store highly salted foods in aluminum containers!
Question. Is it possible to store sour cabbage soup and compote in aluminum containers?
Less active metals, which are in the series of voltages after aluminum, react with water in a highly crushed state and with strong heating (above 100 °C) according to the following scheme:
Metals that are less active than iron do not react with water!
Metals react with salt solutions . In this case, more active metals displace the less active metal from the solution of its salt:
Task 8.9. Which of the following reactions are possible and why:
- silver + copper nitrate II;
- nickel + lead nitrate II;
- copper + mercury nitrate II;
- zinc + nickel nitrate II.
Write down equations for possible reactions. For impossible ones, explain why they are impossible.
It should be noted (!) that very active metals, which under normal conditions react with water, do not displace other metals from solutions of their salts, since they react with water and not with salt:
And then the resulting alkali reacts with salt:
Therefore, the reaction between ferrous sulfate and sodium is NOT accompanied by the displacement of the less active metal:
Melting point table for low-melting metals and alloys:
| Metal name | Melting point, oC |
| Mercury | -38,83 |
| France | 25 |
| Cesium | 28,44 |
| Gallium | 29,7646 |
| Rubidium | 39,3 |
| Potassium | 63,5 |
| Sodium | 97,81 |
| Indium | 156,5985 |
| Lithium | 180,54 |
| Tin | 231,93 |
| Polonium | 254 |
| Bismuth | 271,3 |
| Thallium | 304 |
| Cadmium | 321,07 |
| Lead | 327,46 |
| Zinc | 419,53 |
conclusions
Metals are simple substances that are always reducing agents. The reduction activity of the metal decreases in the voltage series from lithium to gold. By the position of the metal in the stress series, you can determine how the metal reacts with acid solutions, with water, with salt solutions.
Lesson 9. Alkali and alkaline earth metals →
← Lesson 7. The concept of redox reactions
Melting point table for medium-melting metals and alloys:
| Metal name | Melting point, oC |
| Antimony | 630,63 |
| Neptunium | 639 |
| Plutonium | 639,4 |
| Magnesium | 650 |
| Aluminum | 660,32 |
| Radium | 700 |
| Barium | 727 |
| Strontium | 777 |
| Cerium | 795 |
| Ytterbium | 824 |
| Europium | 826 |
| Calcium | 841,85 |
| Lanthanum | 920 |
| Praseodymium | 935 |
| Germanium | 938,25 |
| Silver | 961,78 |
| Neodymium | 1024 |
| Promethium | 1042 |
| Actinium | 1050 |
| Gold | 1064,18 |
| Samarium | 1072 |
| Copper | 1084,62 |
| Uranus | 1132,2 |
| Manganese | 1246 |
| Beryllium | 1287 |
| Gadolinium | 1312 |
| Terbium | 1356 |
| Dysprosium | 1407 |
| Nickel | 1455 |
| Holmium | 1461 |
| Cobalt | 1495 |
| Yttrium | 1526 |
| Erbium | 1529 |
| Iron | 1538 |
| Scandium | 1541 |
| Thulium | 1545 |
| Palladium | 1554,9 |
| Protactinium | 1568 |
Structure
Regardless of activity, all metals have a common structure. Atoms in a simple metal are not arranged chaotically, as in amorphous substances, but in an orderly manner - in the form of a crystal lattice. A metal bond holds the atoms in one position.
This type of connection is carried out due to positively charged ions located at the nodes of a crystal cell (lattice unit), and negatively charged free electrons, which form the so-called electron gas. Electrons separated from atoms, turning them into ions, and began to move randomly in the lattice, holding the ions together. Without electrons, the lattice would disintegrate due to the rejection of equally charged ions.
There are three types of crystal lattice. Body-centered cubic consists of 9 ions and is characteristic of chromium, iron, and tungsten. Face-centered cubic contains 14 ions and is characteristic of lead, aluminum, and silver. A hexagonal close-packed lattice of zinc, titanium, and magnesium consists of 17 ions.
Rice. 2. Types of crystal lattices.
Melting point table for refractory metals and alloys:
| Metal name | Melting point, oC |
| Lutetium | 1652 |
| Titanium | 1668 |
| Thorium | 1750 |
| Platinum | 1768,3 |
| Zirconium | 1855 |
| Chromium | 1907 |
| Vanadium | 1910 |
| Rhodium | 1964 |
| Technetium | 2157 |
| Hafnium | 2233 |
| Ruthenium | 2334 |
| Iridium | 2466 |
| Niobium | 2477 |
| Molybdenum | 2623 |
| Tantalum | 3017 |
| Osmium | 3033 |
| Rhenium | 3186 |
| Tungsten | 3422 |
Position in the periodic table
Metals occupy groups I-II and secondary subgroups of groups III-VIII. Metallic properties, i.e. the ability to donate valence electrons or be oxidized increases from top to bottom as the number of energy levels increases. From left to right, metallic properties weaken, so the most active metals are in groups I-II, the main subgroups. These are alkali and alkaline earth metals.
The degree of activity of metals can be determined by the electrochemical series of voltages. Metals that come before hydrogen are the most active. After hydrogen come weakly active metals that do not react with most substances.
Rice. 1. Electrochemical series of metal voltages.
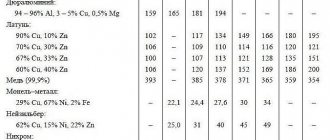
Density:
Depending on their density, metals are divided into light (density from 0.53 to 5 g/cm³) and heavy (from 5 to 22.6 g/cm³).
The lightest metal is lithium (density 0.53 g/cm³). It is currently impossible to name the heaviest metal, since the densities of osmium and iridium - the two heaviest metals - are almost equal (about 22.6 g / cm³ - exactly twice the density of lead), and it is extremely difficult to calculate their exact density: for To do this, the metals must be completely purified, because any impurities reduce their density.
Internal structure and physical properties of metals
Metals are simple substances whose atoms can only give up electrons. This feature of metals is due to the fact that at the outer level of these atoms there are few electrons (most often from 1 to 3) or the outer electrons are located far from the nucleus. The fewer electrons at the outer level of the atom and the further they are located from the nucleus, the more active the metal (the more pronounced its metallic properties).
Task 8.1. Which metal is more active:
Name the chemical elements A, B, C, D.
Metals and non-metals in Mendeleev's Periodic Table of Chemical Elements (PSM) are separated by a line drawn from boron to astatine. Above this line in the main subgroups are non-metals (see lesson 3). The remaining chemical elements are metals.
Task 8.2. Which of the following elements are metals: silicon, lead, antimony, arsenic, selenium, chromium, polonium?
Question. How can we explain the fact that silicon is a non-metal, and lead is a metal, although they have the same number of outer electrons?
An essential feature of metal atoms is their large radius and the presence of valence electrons weakly bound to the nucleus. For such atoms, the ionization energy* is small.
* IONIZATION ENERGY is equal to the work spent on removing one external electron from an atom (to ionize an atom) that is in the ground energy state.
Some of the valence electrons of metals, breaking away from atoms, become “free”. “Free” electrons easily move between atoms and metal ions in the crystal, forming an “electron gas” (Fig. 28).
At a subsequent moment in time, any of the “free” electrons can be attracted by any cation, and any metal atom can give up an electron and turn into an ion (these processes are shown in Fig. 28 by dotted lines).
Thus, the internal structure of a metal is similar to a layer cake, where positively charged “layers” of metal atoms and ions alternate with electronic “layers” and are attracted to them. The best model of the internal structure of a metal is a stack of glass plates moistened with water: it is very difficult to tear one plate from another (strong metals), and it is very easy to move one plate relative to another (ductile metals) (Fig. 29).
Task 8.3. Make such a “model” of the metal and verify these properties.
A chemical bond carried out by “free” electrons is called a metallic bond .
“Free” electrons also provide such physical properties of metals as electrical and thermal conductivity, plasticity (malleability), as well as metallic luster.
Task 8.4. Find metal objects around the house.
By completing this task, you can easily find metal utensils in the kitchen: pots, pans, forks, spoons. Machine tools, airplanes, cars, diesel locomotives, and tools are made from metals and their alloys. Modern civilization is impossible without metals, since electrical wires are also made of metals - Cu and Al. Only metals are suitable for making antennas for radio and television receivers; the best mirrors are made from metals. In this case, not pure metals are often used, but their mixtures (solid solutions) - ALLOYS.
Plastic:
Most metals are ductile, meaning metal wire can be bent without breaking. This occurs due to the displacement of layers of metal atoms without breaking the bond between them.
The most ductile are gold, silver and copper. Gold can be used to make foil 0.003 mm thick, which is used for gilding products. However, not all metals are ductile. Wire made of zinc or tin crunches when bent; When deformed, manganese and bismuth hardly bend at all, but immediately break.
Plasticity also depends on the purity of the metal. Thus, very pure chromium is very ductile, but, contaminated with even minor impurities, it becomes brittle and harder. Some metals, such as gold, silver, lead, aluminum, osmium, can grow together, but this can take decades.
Performance characteristics
Manufacturability is very important in the production process. This is the ability to undergo various types of processing in order to create various products. Technological properties:
Under the influence of external forces, metal can change shape in both heated and cold states. This ability is called malleability. This property was discovered by man in ancient times. Unlike stone, which crumbles and crumbles under strong impacts, metal blanks are easy to process. They can be given a certain shape using a hammer and anvil. This is due to the structure of the crystal lattice.
When heated, two surfaces of metal alloys can be firmly joined to each other, this ability is called weldability.
An important characteristic of molten metal is called fluidity. It characterizes the ability of a material to spread over a prepared form. Each metal can be hardened to a certain depth. This property is called hardenability. The main production operations for obtaining workpieces include processing with cutting tools. Susceptibility to cutting is another property of metal compounds.
Electrical conductivity:
All metals conduct electricity , due to the presence in their crystal lattices of mobile electrons that move under the influence of an electric field.
Silver, copper and aluminum have the highest electrical conductivity. For this reason, the last two metals are most often used as wire materials. Sodium also has very high electrical conductivity. In experimental equipment, attempts are known to use sodium conductors in the form of thin-walled stainless steel pipes filled with sodium. Due to the low specific gravity of sodium, with equal resistance, sodium “wires” are much lighter than copper and even somewhat lighter than aluminum.
Detailed description of mechanical properties
When two bodies come into contact, microdents are formed, but a harder material is able to withstand impacts to a greater extent. The resistance of a metal surface to the penetration of another solid body into it is called hardness.
In addition to hardness, strength is an important characteristic. This is the ability to resist destruction under the influence of any other external forces. With a rapid increase in impact load, the viscosity of the material - this is the ability to resist impact loads. Metal has the property of elasticity. This characteristic allows it to return to its original shape and size after the force is removed. The next concept is plasticity. This term refers to the ability of a metal to change shape under external influence and return to its original state after this influence is removed.