Austenite structure
Austenite
has a g.c.c. γ-Fe structure. The structure of austenite is characterized by the following:
- The lattice parameter of γ-Fe increases linearly from 0.3637 nm at 911°C to 0.368 nm at 1390°C.
- Carbon dissolves in γ-Fe in the form of C4+ and occupies octapores, forming interstitial solid solutions.
- With increasing carbon content in austenite
, the lattice period of the γ-phase increases.
Crystal structure of austenite
can be thought of as a face-centered lattice consisting of iron atoms, into which smaller carbon atoms are embedded. Since the carbon atom is larger than the size of the pore (free space in the fcc lattice), when it enters the iron lattice, the latter is distorted and this makes the remaining pores inaccessible to other carbon atoms. The structure of austenite can be stabilized by alloying, since all elements that dissolve in iron affect the temperature range of existence of its allotropic modifications, in particular austenite.
In other words, the structure of austenite
is obtained when the steel contains a high content of an alloying element (Ni, Mn, etc.), expanding the region of the γ-phase.
For more information, see the Austenitic steels page.
©ICM (www.modificator.ru)
Properties of austenitic steels and where they are used
The very state of iron in the Y-phase (austenite) is unique, thanks to which the metal is heat-resistant (+850 ºC), cold-resistant (-100 ºC and below t), capable of providing corrosion and electrochemical resistance and other important properties, without which many would be unthinkable technological processes in:
- oil refining and chemical industries;
- medicine;
- space and aircraft construction;
- electrical engineering.
Heat resistance is the property of steel not to change its technical properties at critical temperatures over time. Fracture occurs when the metal is unable to resist dislocation creep, i.e., the displacement of atoms at the molecular level. Softening gradually occurs, and the aging process of the metal begins to occur faster and faster. This occurs over time at low or high temperatures. So, the extent to which this process extends over time is the metal’s ability to resist heat.
Corrosion resistance is the ability of a metal to resist destruction (dislocation creep) not only over time and at cryogenic and high temperatures, but also in aggressive environments, that is, when interacting with substances that actively react with one or more component elements. There are 2 types of corrosion:
- chemical - oxidation of metal in environments such as gas, water, air;
- electrochemical - dissolution of a metal in acidic environments containing positively or negatively charged ions. When there is a potential difference between the metal and the electrolyte, inevitable polarization occurs, leading to partial interaction of the two substances.
Cold resistance - the ability to maintain structure at cryogenic temperatures over a long period of time. Due to the distortion of the crystal lattice, the structure of cold-resistant steel is capable of taking on the structure inherent in ordinary low-alloy steels, but at very low temperatures. But these steels have one drawback - they can have full properties only at subzero temperatures; t - ≥ 0 is unacceptable for them.
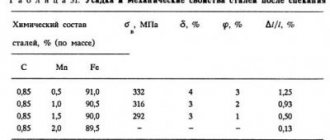
Retained austenite
The typical martensite structure of hardened steel has a needle-like appearance. Austenite, which exists at normal temperature along with martensite, is called retained austenite.
.
In the presence of significant amounts of retained austenite (practically more than 20-30%), it is detected metallographically in the form of bright fields between martensite needles. The amount of retained austenite
in steel, fixed by quenching, depends on the position of the martensite point.
The lower the martensite point, the more retained austenite. Therefore, carbon, by lowering the martensite point, increases the amount of retained austenite
. Hardened low-carbon steels contain almost no retained austenite (content of retained austenite in steels). The presence of retained austenite in the structure of martensitic (hardened) steel reduces hardness and is an undesirable phenomenon.
When heated, retained martensite decomposes with different scenarios, depending on the initial amount of retained austenite, as well as the degree of carbon concentration in the retained austenite (Transformations in steels during tempering, [4]).
Application of alloys
Austenitic steels are used in the manufacture of devices operating at high temperatures, starting from 200 °C: steam generators, rotors, turbines and welding mechanisms. The disadvantage of using austenite in these mechanisms is the low strength of the metal. With prolonged contact of iron alloys with various hydroxides, additional cracks may form, which will lead to breakage of the working surfaces of devices. This drawback can be eliminated by adding additional chemical elements to the iron solution: vanadium and niobium. They form a carbide phase, which increases the strength of steel.
Stainless austenitic steels are used in mechanisms operating in difficult conditions and with strong temperature changes. They are most often used when welding corrosion-resistant pipes. During this process, a seam space is created between the fasteners. When stainless austenite pipes are heated to the melting temperature, they acquire a monolithic structure that protects the metal from oxidation processes and high temperature changes.
Austenite hardness
The hardness of austenite is influenced by various factors, primarily the content of dissolved carbon (and other alloying elements that form substitutional solid solutions), therefore there cannot be a clear and unique value of austenite hardness (only the order of values of austenite hardness is known). Therefore, austenite hardness values are usually indicated within a certain range, and therefore we find slightly different austenite hardness values in different sources. For example, according to [5], the Brinell hardness of austenite is 160-200 HB.
During metallographic analysis in each specific case (alloy, casting), it is desirable to determine the hardness of austenite experimentally, collecting additional statistical information (see Hardness, Microhardness).
©ICM (www.modificator.ru)
Types of austenitic steels
Based on their composition and physical properties, there are 3 types of austenite steel:
Anti-corrosion austenitic grade steel
This category includes alloys with a high specific content of chromium and nickel. The alloy may also contain silicon, manganese, and molybdenum in small quantities. The peculiarity of alloys of this group is the minimal risk of corrosion at any temperature.
High stability is ensured due to two factors. The first factor is the high chromium content, which creates a protective film on the surface of the steel. The second factor is low carbon content (less than 0.3%). The combination of these factors leads to the fact that the material does not come into contact with oxygen, nitrogen, water, and various chemicals.
Stability is maintained even when heated or cooled, since chromium retains its physical properties when temperatures change.
Heat resistant class
This category includes alloys with a high content of nickel, boron, niobium, vanadium, molybdenum, and tungsten. Alloying components make the material more durable and minimize the risk of pores forming between individual iron atoms. Therefore, heat-resistant austenite retains its shape when heated to 1100 degrees.
Heat-resistant austenite material is suitable for the manufacture of various furnaces, machine tools, and factory equipment. Some alloys also contain large amounts of chromium. The result is a heat-resistant anti-corrosion alloy that not only withstands heat, but also does not corrode.
Cold resistant class
This category includes alloys with a high specific chromium content and an average nickel content. Aluminum, manganese, vanadium, and tungsten can be used as additional alloying additives.
Cold-resistant alloys can withstand very low temperatures and withstand sudden temperature changes. However, at normal room temperature, cold-resistant austenite steel has mediocre physical properties - low strength, weak chemical inertness.
Therefore, cold-resistant alloys are used to make special equipment for regions with very cold climates. Another area of application is the manufacture of parts, products, and equipment for the needs of the space industry.
Nitrogen in austenitic stainless steels
Nitrogen, like carbon, has variable solubility in austenite. Nitrogen can form independent nitride phases during cooling and isothermal exposure or be part of carbides, replacing carbon in them. The effect of nitrogen on the susceptibility to intergranular corrosion of chromium-nickel austenitic steels is much weaker than that of carbon, and begins to appear only when its content is more than 0.10-0.15%. At the same time, the introduction of nitrogen increases the strength of chromium-nickel austenitic steel. Therefore, in practice, small additions of nitrogen are used in these steels.
Resistance of austenitic chromium-nickel steels to acids
The ability to passivate provides chromium-nickel austenitic steels with fairly high resistance to nitric acid. Steels 12Х18Н10Т, 12Х18Н12Б and 02Х18Н11 have the first resistance rating:
- in 65% nitric acid at temperatures up to 85 ºС;
- in 80% nitric acid at temperatures up to 65 ºС;
- 100% sulfuric acid at temperatures up to 65 ºС;
- in mixtures of nitric and sulfuric acids: (25% + 70%) and 10% + 60%) at temperatures up to 70 ºС;
- in 40% phosphoric acid at 100 ºС.
Austenitic chromium-nickel steels also have high resistance to solutions of organic acids - acetic, citric and formic, as well as alkalis KOH and NaOH.
Welding austenitic steel
Welding technology can be used to connect austenite products. The joining of metals can be carried out by all main welding methods (electroslag, arc, gas-shielded).
Welding austenitic steels has many features and nuances that the welder needs to know about in advance. The peculiarity is a serious change in the physical properties of the austenite metal when heated. This imposes a number of requirements regarding welding. After all, if the metal is heated incorrectly, the quality of the weld seriously suffers, which will have a bad effect on the strength of the connection.
Features of austenite heating
- At a temperature of +350 degrees, active diffusion processes occur in the alloy, which leads not to an increase, but to a decrease in the ductility of the metal.
- From +350 to +500 degrees, thermal restructuring of the metal occurs. Such a physical process has a number of characteristic features - increased fragility of the material, cracking of carbide components, and changes in thermal conductivity.
- From +500 to +650 degrees, precipitation of carbide components occurs, which the welder must take into account during work.
- When the material is heated above +750 degrees, the fragility of the metal seriously increases. With such heating, small cracks can form on the metal, which reduces the strength of the weld.
However, the welder must avoid the appearance of cracks, irregularities, and holes in the weld area. To solve this problem, a small metal layer is fused onto the part in the weld area, which has a different chemical composition.
The patch layer requires a metal with increased heat resistance and high corrosion resistance. The patch will act as a protective layer that will prevent the seam from cracking. It is recommended to fire the protective layer at a temperature of +800 degrees to avoid the appearance of cracks under increased load levels.
Electroslag welding
Electroslag welding technology is suitable for joining both large and small austenite-based products. The main advantages of this technology are the minimal risk of cracks, the absence of deformation at the joints, and the ease of welding.
Welding is recommended to be carried out quickly and at low temperatures. Indeed, when the metal is heated for a long time above a temperature of 1200 degrees, local cracks can form, which can lead to the destruction of the metal.
A few additional notes regarding the use of electroslag technology:
- Welding is recommended to be done using wire, the thickness of which is 2-4 millimeters. The main disadvantage of this approach is that high-quality wire is consumed quickly, and it is quite expensive.
- To connect thick parts, plate electrodes should be used (optimal thickness - 5-15 millimeters). Electrodes are more expensive, but they degrade much more slowly.
- When working with alloys that have increased corrosion resistance, it is recommended to do hardening or annealing - this will help avoid the appearance of knife corrosion.
Arc welding
Arc welding for joining austenitic steel has many disadvantages.
Main disadvantage:
- During welding, the local region of the austenite metal is heated. Heat does two dangerous things that negatively affect strength.
- The first point is the appearance of iron oxides in the weld area. The physics of this process is as follows: when seriously heated, iron begins to come into contact with atmospheric air, which leads to the formation of oxides.
- The second point is the appearance of cracks near the seam. With high heating, the fragility of the material sharply increases while the overall ductility decreases, which contributes to the formation of small cracks.
Calcium fluoride electrodes
There are a number of techniques that can overcome the limitations of arc welding. The most popular method is the use of calcium fluoride electrodes of small diameter (the optimal cross-sectional diameter is 3-5 millimeters).
Such rods have low ductility, so during welding work the electrodes do not make unnecessary vibrations. This reduces the contact of the molten metal with air, and also reduces the risk of cracks due to increased brittleness.
1.5-2 hours before welding, it is recommended to calcinate calcium fluoride electrodes at a low temperature (200-300 degrees). This helps minimize the risk of porosity in the electrode.
Electric arc welding must be performed strictly with reverse polarity direct current. Otherwise, the stability of the electrode is not guaranteed.
Gas shielded welding
Welding austenitic steels using shielding gases is the best way to join austenites. This technique allows you to connect parts of various shapes, and welding can be carried out in any spatial position.
The use of shielding gases minimizes the likelihood of cracks, plaque, rust, and scale, which makes the welded joint very durable. Any gas can be used as a protective medium - argon, helium, nitrogen, carbon dioxide and others. For welding, consumable or tungsten rods are usually used, which are suitable for creating small, strong seams (the optimal thickness is 5-10 millimeters).
Features of austenite welding in shielding gases
- For welding work, you can use both a pulsed and a burning arc. However, experienced welders recommend using a pulsed arc. This reduces the thickness of the seam and minimizes the likelihood of edge crushing. Thanks to this, it is possible to obtain an even, strong seam that will not crack during long-term use of the product.
- It is recommended to weld austenite using direct current, which has straight polarity. If necessary, the polarity of the current can be changed - this will not affect the quality of the weld in any way. When choosing a burner, you need to pay attention to the type of polarity switching. After all, most burners work with devices that switch polarity automatically. If you want to change the polarity manually, you must read the instructions for the burner to make sure that it supports this mode of operation. Also note that when welding austenite with a high content of aluminum fillers, it is recommended to use an AC torch.
- For pulsed arc welding, it is recommended to use consumable electrodes. This connection method is suitable for connecting structures with a small thickness. These can be metal sheets, thin beams, and so on. The use of a consumable electrode minimizes the risk of cracks in the seam, which will have a beneficial effect on the shelf life of such a welded joint.
- Plasma welding of austenitic steels is permitted in situations where the thickness of individual welded elements is less than 15 millimeters. In the case of plasma welding of large objects, the risk of formation of undercuts and gaps increases sharply, which negatively affects the strength of the welded joint.
Delta Ferrite in Chromium Molybdenum Austenitic Steel
The presence of delta ferrite in the structure of austenitic chromium-nickel steel type 18-10 has a negative effect on its manufacturability during hot plastic deformation - rolling, piercing, forging, stamping.
The amount of ferrite in steel is strictly limited by the ratio of chromium and nickel in it, as well as by technological means. The group of steels most prone to the formation of delta ferrite is the X18N9T type (see also Stainless steels). When these steels are heated to 1200 ºС, the structure can contain up to 40-45% delta ferrite. The most stable are steels of the X18N11 and X18N12 types, which, when heated at high temperatures, retain an almost purely austenitic structure.
Stabilizing annealing of austenitic chromium-nickel steels
In unstabilized steels, annealing is carried out in the temperature range between the heating temperature for hardening and the maximum temperature for the manifestation of intergranular corrosion. The value of this interval primarily depends on the chromium content in the steel and increases with increasing its concentration.
In stabilized steels, annealing is carried out to transfer carbon from chromium carbides to special titanium and niobium carbides. In this case, the released chromium goes to increase the corrosion resistance of steel. The annealing temperature is usually 850-950 ºС.