4.5
Average rating: 4.5
Total ratings received: 185.
4.5
Average rating: 4.5
Total ratings received: 185.
Metals are a special group of elements in the periodic table. Unlike non-metals, the elements of this group are exclusively reducing agents with a positive oxidation state, and also have plasticity, hardness, and elasticity, which is due to the crystalline structure of metals.
Molecular kinetic theory
All molecules are made up of tiny particles called atoms. All currently discovered atoms are collected in the periodic table.
An atom is the smallest, chemically indivisible particle of a substance that retains its chemical properties. Atoms are connected to each other by chemical bonds . Previously, we already looked at the types of chemical bonds and their properties. Be sure to study the theory on the topic: Types of Chemical Bonds before studying this article!
Now let's look at how particles in matter can connect.
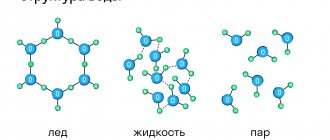
Depending on the location of the particles relative to each other, the properties of the substances they form can vary greatly. far from each other (the distance between the particles is much greater than the size of the particles themselves), practically do not interact with each other, and move in space chaotically and continuously, then we are dealing with a gas .
If the particles are located close to each other, but chaotically , interact more with each other , perform intense oscillatory movements in one position, but can jump to another position, then this is a model of the structure of a liquid .
If the particles are located close to each other, but in a more orderly manner , and interact more with each other, and move only within one equilibrium position, practically without moving to other positions, then we are dealing with a solid substance .
Most known chemical substances and mixtures can exist in solid, liquid and gaseous states. The simplest example is water. Under normal conditions it is liquid , at 0 oC it freezes - it goes from a liquid to a solid , and at 100 oC it boils - it goes into the gas phase - water vapor. Moreover, many substances under normal conditions are gases, liquids or solids. For example, air - a mixture of nitrogen and oxygen - is a gas under normal conditions. But at high pressure and low temperature, nitrogen and oxygen condense and pass into the liquid phase. Liquid nitrogen is actively used in industry. Sometimes plasma , as well as liquid crystals, as separate phases.
Many properties of individual substances and mixtures are explained by the relative arrangement of particles in space relative to each other!
This article examines the properties of solids , depending on their structure. Basic physical properties of solids: melting point, electrical conductivity, thermal conductivity, mechanical strength, plasticity, etc.
Melting point is the temperature at which a substance changes from solid to liquid and vice versa.
Plasticity is the ability of a substance to deform without breaking.
Electrical conductivity is the ability of a substance to conduct current.
Current is the ordered movement of charged particles . Thus, only substances that contain mobile charged particles . Based on their ability to conduct current, substances are divided into conductors and dielectrics. Conductors are substances that can conduct current (i.e. contain mobile charged particles). Dielectrics are substances that practically do not conduct current.
In a solid matter, the particles of a substance can be arranged randomly or more ordered . randomly located in space , the substance is called amorphous . Examples of amorphous substances are coal, mica glass.
Amorphous boron
If the particles of a solid substance are arranged in space in an orderly manner, i.e. form repeating three-dimensional geometric structures, such a substance is called a crystal , and the structure itself is called a crystal lattice . Most of the substances we know are crystals. The particles themselves are located at the nodes of the crystal lattice.
Crystalline substances are distinguished, in particular, by the type of chemical bond between particles in the crystal - atomic, molecular, metallic, ionic; according to the geometric shape of the simplest cell of a crystal lattice - cubic, hexagonal, etc.
Depending on the type of particles that form the crystal lattice, atomic, molecular, ionic and metallic crystal structures are distinguished.
The world of metals through the eyes of a chemist. 9th grade. Lesson development
Lesson 2 “The world of metals through the eyes of a chemist” is the initial stage of studying the topic “Metals” (24 hours) according to the O.S. program. Gabrielyan.
There are several students in the class who are interested in chemistry; the subject is attractive to them due to its practical orientation, variety of activities, and clarity when studying the material.
Lesson type: learning new material
Lesson type: research lesson
Lesson objectives: to study the position of metals in P.S.H.E., to reveal the reasons for the general physical properties of metals.
Types of cognitive activity: Statement of a problem, observation of an experiment, the ability to highlight the main thing, compare, generalize, logically express thoughts.
Methods of managing cognitive activity: Familiarization with the goals and objectives of the lesson, type of control.
Forms of organizing educational activities: individual and group activities, laboratory experience.
Methods for organizing educational activities : conversation, demonstration experiment, problem situation method.
Means of education:
- Textbooks: O.S. Gabrielyan "Chemistry" 9th grade.
- Collections of metal samples, aluminum foil, iron nail, alcohol lamp, Christmas tree decoration, mirror, glass plates, periodic table.
- TSO tools: computer, projector, screen.
- Software: Power Point.
Lesson steps
Organizational moment. Introduction.
Interesting facts-mysteries about metals: (slide No. 2)
- This element is especially needed by a growing child's body. In an adult, without it, bones break, blood does not clot, and the heart does not work well. A shellfish won’t build a house without it, a turtle will be left without a roof, and a chicken and an egg will have nothing to pack it in. (Calcium)
- If you break the thermometer, do not play with the shiny drop. Its fumes are poisonous. (Mercury)
- The name of this chemical element is translated from the ancient Armenian language as “dropped from the sky,” but it no longer drips from the sky onto us, although our body needs it. (Iron)
- The substance formed by this chemical element has a bactericidal effect. It is known that in ancient times they stored water in vessels made from this substance, so it did not spoil for a long time. (Silver)
- The substance formed by this chemical element is extremely chemically stable and at the same time compatible with human tissue. Therefore, it is indispensable in reconstructive surgery. (Tantalum)
Teacher question: Which group of chemical elements do those listed on the slide belong to? (metals)
State the topic and purpose of the lesson.
II. Preparation for the main stage of mastering educational material.
Alchemists believed that “light created seven metals according to the number of seven planets.” Name these elements and their corresponding planets. (Textbook p. 22)
After listening to the answers, we read an excerpt from the alchemist’s notes (translation by N. Morozov):
Seven metals were created by light According to the number of seven planets: The cosmos gave us for good copper, iron, silver, Gold, tin, lead... My son. Sera is their father. And hasten, my son, to find out: Mercury is their own mother to all of them.
Teacher question: What does the word Metal mean? What is its meaning? (the word metal can mean a chemical element and a simple substance.)
Scheme:
Teacher: What is a chemical element? (set of atoms) Together with students, we find out the structural features of metal atoms based on their position in the PSHE.
Teacher: If you draw a diagonal from B to At through the elements of the main subgroups, then along this diagonal (B-Si-As-Te-At) and above it there will be non-metals, and below it - metals. As a result, out of 110 PS elements, 88 elements belong to metals. However, the division of elements into metals and non-metals is arbitrary. For example, the metal germanium has many non-metallic properties. Chromium, aluminum and zinc are typical metals, but they form compounds in which they exhibit non-metallic properties: NaAlO2, K2ZnO2, K2CrO4, K2Cr2O7. From the position of metals in the PS, it is possible to determine the structural features of their atoms: (slide No. 3)
- A small number of electrons in the outer level.
- Relatively large atomic radius
- The ability to donate external electrons and exhibit reducing properties. (Problem: Why does boron, whose atoms have three electrons in the outer shell, exhibit typical nonmetallic properties?)
Atomic crystal lattice
An atomic crystal lattice is formed when atoms . The atoms are connected to each other by strong covalent chemical bonds . Accordingly, such a crystal lattice will be very strong and not easy to destroy. An atomic crystal lattice can be formed by atoms with high valency, i.e. with a large number of bonds with neighboring atoms (4 or more). As a rule, these are non-metals: simple substances - silicon, boron, carbon (allotropic modifications diamond, graphite), and their compounds (borocarbon, silicon oxide (IV), etc.). Since a predominantly covalent chemical bond occurs between nonmetals, in most cases there are no free electrons (as well as other charged particles) in substances with an atomic crystal lattice . Consequently, such substances, as a rule, conduct electricity very poorly, i.e. are dielectrics . These are general patterns, to which there are a number of exceptions.
Bonding between particles in atomic crystals: covalent polar or nonpolar .
Atoms at the nodes of a crystal with an atomic crystal structure .
Phase state of atomic crystals under normal conditions: usually solids .
Substances that form atomic crystals in the solid state:
- Simple substances with high valency (located in the middle of the periodic table): boron, carbon, silicon, etc.
- Complex substances formed by these non-metals: silica (silicon oxide, quartz sand) SiO2; silicon carbide (carborundum) SiC; boron carbide, boron nitride, etc.
Physical properties of substances with an atomic crystal lattice:
- strength;
— refractoriness (high melting point);
— low electrical conductivity;
— low thermal conductivity;
— chemical inertness (inactive substances);
- insolubility in solvents.
APPLICATION
Iron is one of the most used metals, accounting for up to 95% of global metallurgical production.
Iron is the main component of steels and cast irons - the most important structural materials.
Iron can be part of alloys based on other metals - for example, nickel. Magnetic iron oxide (magnetite) is an important material in the production of long-term computer memory devices: hard drives, floppy disks, etc.
Ultrafine magnetite powder is used in many black and white laser printers mixed with polymer granules as a toner. This uses both the black color of the magnetite and its ability to stick to the magnetized transfer roller.
The unique ferromagnetic properties of a number of iron-based alloys contribute to their widespread use in electrical engineering for magnetic cores of transformers and electric motors.
Iron(III) chloride (ferric chloride) is used in amateur radio practice for etching printed circuit boards.
Ferrous sulfate heptate (ferrous sulfate) mixed with copper sulfate is used to combat harmful fungi in gardening and construction.
Iron is used as an anode in iron-nickel batteries and iron-air batteries.
Aqueous solutions of ferrous and ferric chlorides, as well as its sulfates, are used as coagulants in the purification processes of natural and waste water in the water treatment of industrial enterprises.
Molecular crystal lattice
A molecular crystal lattice is a lattice in which molecules . Molecules are held in a crystal by weak forces of intermolecular attraction (van der Waals forces, hydrogen bonds, or electrostatic attraction). Accordingly, such a crystal lattice is usually quite easy to destroy . Substances with a molecular crystal lattice are fusible and fragile . The greater the force of attraction between molecules, the higher the melting point of the substance . As a rule, the melting temperatures of substances with a molecular crystal lattice are not higher than 200-300K. Therefore, under normal conditions, most substances with a molecular crystal lattice exist in the form of gases or liquids . A molecular crystal lattice is usually formed in solid form by acids, non-metal oxides, other binary compounds of non-metals, simple substances that form stable molecules (oxygen O2, nitrogen N2, water H2O, etc.), organic substances. As a rule, these are substances with a covalent polar (less often nonpolar) bond. Because electrons are involved in chemical bonds, substances with a molecular crystal lattice are dielectrics and conduct heat poorly .
Communication between particles in molecular crystals: intermolecular hydrogen bonds, electrostatic or intermolecular forces of attraction .
Molecules at the nodes of a crystal with a molecular crystal structure .
Phase state of molecular crystals under normal conditions: gases, liquids and solids .
Substances that form molecular crystals :
- Simple non-metallic substances that form small strong molecules (O2, N2, H2, S8, etc.);
- Complex substances (non-metal compounds) with covalent polar bonds (except for silicon and boron oxides, silicon and carbon compounds) - water H2O, sulfur oxide SO3, etc.
- Monatomic noble gases ( helium, neon, argon, krypton, etc.) ;
- Most organic substances that do not have ionic bonds are methane CH4, benzene C6H6, etc.
Physical properties of substances with a molecular crystal lattice:
— fusibility (low melting point):
— high compressibility;
— molecular crystals in solid form, as well as in solutions and melts, do not conduct current;
- phase state under normal conditions - gases, liquids, solids;
— high volatility;
- low hardness.
Defects in the crystal structure of metals
However, all types of cells considered may also have natural shortcomings, or so-called defects. This may be due to various reasons: foreign atoms and impurities in metals, external influences, and so on.
Therefore, there is a classification that reflects the defects that crystal lattices may have. Chemistry as a science studies each of them in order to identify the cause and method of elimination so that the properties of the material are not changed. So, the defects are as follows.
- Spot. They come in three main types: vacancies, impurities or dislocated atoms. They lead to deterioration of the magnetic properties of the metal, its electrical and thermal conductivity.
- Linear or dislocation. There are edge and screw ones. They deteriorate the strength and quality of the material.
- Surface defects. Affects the appearance and structure of metals.
Currently, methods have been developed to eliminate defects and obtain pure crystals. However, it is not possible to completely eradicate them; an ideal crystal lattice does not exist.
Ionic crystal lattice
If there are charged particles - ions , we can talk about an ionic crystal lattice . Typically, ionic crystals alternate between positive ions (cations) and negative ions (anions), so the particles in the crystal are held together by forces of electrostatic attraction . Depending on the type of crystal and the type of ions that form the crystal, such substances can be quite strong and refractory . In the solid state, there are usually no mobile charged particles in ionic crystals. But when the crystal dissolves or melts, the ions are released and can move under the influence of an external electric field. Those. Only solutions or melts of ionic crystals conduct current. An ionic crystal lattice is characteristic of substances with ionic chemical bonds . Examples of such substances are table salt NaCl, calcium carbonate - CaCO3, etc. An ionic crystal lattice, as a rule, is formed in the solid phase by salts, bases, as well as metal oxides and binary compounds of metals and non-metals .
Bonding between particles in ionic crystals: ionic chemical bonding.
Ions at the nodes of a crystal with an ionic lattice .
Phase state of ionic crystals under normal conditions: usually solids .
Chemicals with ionic crystal lattice:
- Salts (organic and inorganic), including ammonium salts (for example, ammonium chloride NH4Cl);
- Grounds;
- Metal oxides;
- Binary compounds containing metals and non-metals.
Physical properties of substances with an ionic crystal structure:
— high melting point (refractoriness);
— solutions and melts of ionic crystals are current conductors;
— most compounds are soluble in polar solvents (water);
- solid phase state for most compounds under normal conditions.
The importance of knowledge about the crystalline structure of metals
From the above material, it is obvious that knowledge about the fine structure and structure makes it possible to predict the properties of the material and influence them. And the science of chemistry allows you to do this. The 9th grade of a general education school places emphasis in the learning process on developing in students a clear understanding of the importance of the fundamental logical chain: composition - structure - properties - application.
Read also: Which cable to use for spotlights
Information about the crystalline structure of metals very clearly illustrates this dependence and allows the teacher to clearly explain and show children how important it is to know the fine structure in order to correctly and competently use all the properties.
Content:
Metal crystal lattice
And finally, metals are characterized by a special type of spatial structure - a metal crystal lattice , which is caused by a metallic chemical bond . Metal atoms hold valence electrons rather weakly. In a crystal formed by a metal, the following processes occur simultaneously: some of the atoms give up electrons and become positively charged ions; these electrons move randomly in the crystal; Some electrons are attracted to the ions. These processes occur simultaneously and chaotically. Thus, ions are created , as in the formation of an ionic bond, and shared electrons are formed , as in the formation of a covalent bond. Free electrons move randomly and continuously throughout the entire volume of the crystal, like a gas. Therefore, they are sometimes called " electron gas ". Due to the presence of a large number of mobile charged particles, metals conduct current and heat . The melting point of metals varies greatly. Metals are also characterized by a peculiar metallic luster, malleability , i.e. the ability to change shape without destruction under strong mechanical stress, because chemical bonds are not destroyed.
Bonding between particles : metallic chemical bonding.
Metal ions and atoms at the nodes of a crystal with a metal lattice .
Phase state of metals under normal conditions: usually solids (with the exception of mercury, liquid under normal conditions).
Chemical substances with a metal crystal lattice are simple metal substances .
Physical properties of substances with a metal crystal lattice:
— high thermal and electrical conductivity;
— malleability and plasticity;
- metallic luster;
- metals are usually insoluble in solvents;
- Most metals are solids under normal conditions.
RESERVES AND PRODUCTION
Iron is one of the most common elements in the solar system, especially on the terrestrial planets, in particular on Earth. A significant part of the iron of the terrestrial planets is located in the cores of the planets, where its content is estimated to be about 90%. The iron content in the earth's crust is 5%, and in the mantle about 12%.
Iron is quite widespread in the earth's crust - it accounts for about 4.1% of the mass of the earth's crust (4th place among all elements, 2nd among metals). In the mantle and earth's crust, iron is concentrated mainly in silicates, while its content is significant in basic and ultrabasic rocks, and low in acidic and intermediate rocks.
A large number of ores and minerals containing iron are known. Of greatest practical importance are red iron ore (hematite, Fe2O3; contains up to 70% Fe), magnetic iron ore (magnetite, FeFe2O4, Fe3O4; contains 72.4% Fe), brown iron ore or limonite (goethite and hydrogoethite, respectively FeOOH and FeOOH nH2O ). Goethite and hydrogoethite are most often found in weathering crusts, forming so-called “iron hats”, the thickness of which reaches several hundred meters. They can also be of sedimentary origin, falling out of colloidal solutions in lakes or coastal areas of the seas. In this case, oolitic, or legume, iron ores are formed. Vivianite Fe3(PO4)2·8H2O is often found in them, forming black elongated crystals and radial aggregates.
The iron content in sea water is 1·10−5-1·10−8%
Industrially, iron is obtained from iron ore, mainly hematite (Fe2O3) and magnetite (FeO Fe2O3).
There are various ways to extract iron from ores. The most common is the domain process.
The first stage of production is the reduction of iron with carbon in a blast furnace at a temperature of 2000 °C. In a blast furnace, carbon in the form of coke, iron ore in the form of agglomerate or pellets, and flux (such as limestone) are fed from above, and are met by a stream of forced hot air from below.
In addition to the blast furnace process, the process of direct iron production is common. In this case, pre-crushed ore is mixed with special clay, forming pellets. The pellets are fired and treated in a shaft furnace with hot methane conversion products, which contain hydrogen. Hydrogen easily reduces iron without contaminating the iron with impurities such as sulfur and phosphorus, which are common impurities in coal. Iron is obtained in solid form and is subsequently melted in electric furnaces. Chemically pure iron is obtained by electrolysis of solutions of its salts.
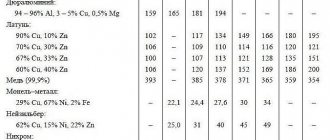
Comparison of the properties of substances with different crystal lattices
The type of crystal lattice (or lack of a crystal lattice) allows one to evaluate the basic physical properties of a substance . To roughly compare the typical physical properties of compounds with different crystal lattices, it is very convenient to use chemicals with characteristic properties. For a molecular lattice this is, for example, carbon dioxide, for an atomic crystal lattice - diamond, for a metal lattice - copper, and for an ionic crystal lattice - table salt, sodium chloride NaCl.
Summary table on the structures of simple substances formed by chemical elements from the main subgroups of the periodic table (elements of the side subgroups are metals, therefore, have a metallic crystal lattice).
The final table of the relationship between the properties of substances and their structure:
General concept of metals
"Chemistry. 9th grade" is a textbook used by schoolchildren. It is here that metals are studied in detail. A large chapter is devoted to the consideration of their physical and chemical properties, because their diversity is extremely great.
Read also: Transistor comparator circuit
It is from this age that it is recommended to give children an idea of these atoms and their properties, because teenagers can already fully appreciate the significance of such knowledge. They see perfectly well that the variety of objects, machines and other things around them is based on a metallic nature.
What is metal? From the point of view of chemistry, these atoms are usually classified as those that have:
- a small number of electrons at the outer level;
- exhibit strong restorative properties;
- have a large atomic radius;
- As simple substances, they have a number of specific physical properties.
The basis of knowledge about these substances can be obtained by considering the atomic-crystalline structure of metals. It is this that explains all the features and properties of these compounds.
In the periodic table, most of the entire table is allocated to metals, because they form all the secondary subgroups and the main ones from the first to the third group. Therefore, their numerical superiority is obvious. The most common are:
All metals have a number of properties that allow them to be combined into one large group of substances. In turn, these properties are explained precisely by the crystalline structure of metals.