How it was opened
People's acquaintance with tungsten dates back to the Middle Ages.
Prospectors
Tungsten was also obtained by European miners during the recovery of tin. But it was considered “garbage” clogging a valuable element. Under the influence of tungsten ore during the reduction process, part of the tin turned into slag, reducing the proportion of the pure substance.
Hence the saying that appeared among prospectors: “Tungsten eats tin like a wolf eats a lamb.”
The science
The history of the discovery of tungsten is associated with several chemists:
- In the mid-18th century, Swede Axel Frederik Kronstedt discovered a heavy metal, which he called Tung Sten (in Swedish - heavy stone).
- 30 years later, his compatriot, member of the country’s Academy of Sciences Karl Scheele, took up the matter. In his free time from working at the pharmacy, he devoted his time to experiments in his home laboratory. He is considered the “father” of not only tungsten. The list also includes barium, manganese, oxygen, and chlorine. From tungsten ore (tungsten) he isolated an acid salt that was not listed in the registers.
- He entrusted further work on the connection to his Spanish colleagues, the Eluard brothers. Which received a new element.
The name and symbol of the metal – Wolframium and W – were proposed by Jens Jakob Berzelius.
The etymology of the name tungsten has German roots: Wolf Rahm (“wolf cream”).
And tungsten was renamed in honor of the scientist - scheelite.
As presented in nature
The native non-ferrous metal tungsten is not found on the planet. It is presented in the form of ore or minerals.
Ores consist of compounds of tungsten with iron, manganese, calcium, and sometimes other elements, including rare earths.
Minerals are inclusions in granites (up to 2%). Of these, wolframite (tungsten with iron and manganese) and scheelite (with calcium) are of industrial importance.
Each ton of the earth's crust contains 1.30 g of tungsten.
Application
Rarity, unusualness and importance determine the widespread use in modern technology of a metal called Tungsten - tungsten. Properties and application justify the high cost and demand. High melting point, hardness, strength, heat resistance and resistance to chemical influences and corrosion, wear resistance and cutting features are its main trump cards. Usage options:
- Filaments.
- Alloying of steels in order to obtain high-speed, wear-resistant, heat-resistant and heat-resistant iron-carbon alloys, which are used for the production of drills and other tools, punches, springs and springs, rails.
- Production of “powdered” hard alloys, used mainly as particularly wear-resistant cutting, drilling or pressing tools.
- Electrodes for argon arc and resistance welding.
- Manufacturing of parts for X-ray and radio equipment, various technical lamps.
- Special luminous paints.
- Wire and parts for the chemical industry.
- Various practical small things, for example, jigs for fishing.
Various alloys containing tungsten are gaining popularity. The scope of application of such materials is sometimes surprising - from heavy engineering to light industry, where fabrics with special properties (for example, fire-resistant) are made.
There are no universal materials. Each known element and created alloys are distinguished by their uniqueness and necessity for certain areas of life and industry. However, some of them have special properties that make previously impossible processes possible. One such metal is tungsten. Its use is not widespread enough, like steel, but each of the options is extremely useful and necessary for humanity.
Physico-chemical characteristics
Pure tungsten is among the first in density, hardness, and first in melting and boiling points among metals. These physical properties are complemented by chemical resistance even at extreme temperatures.
| Properties of the atom | |
| Name, symbol, number | Tungsten / Wolframium (W), 74 |
| Atomic mass (molar mass) | 183.84(1) a. e.m. (g/mol) |
| Electronic configuration | [Xe] 4f14 5d4 6s2 |
| Atomic radius | 137 pm |
| Chemical properties | |
| Covalent radius | 170 pm |
| Ion radius | (+6e) 62 (+4e) 70 pm |
| Electronegativity | 2.3 (Pauling scale) |
| Electrode potential | W ← W3+ 0.11 V W ← W6+ 0.68 V |
| Oxidation states | +2, +3, +4, +5, +6 |
| Ionization energy (first electron) | 769.7 (7.98) kJ/mol (eV) |
| Thermodynamic properties of a simple substance | |
| Density (at normal conditions) | 19.25 g/cm³ |
| Melting temperature | 3695 K (3422 °C, 6192 °F) |
| Boiling temperature | 5828 K (5555 °C, 10031 °F) |
| Ud. heat of fusion | 285.3 kJ/kg 52.31 kJ/mol |
| Ud. heat of vaporization | 4482 kJ/kg 824 kJ/mol |
| Molar heat capacity | 24.27 J/(K mol) |
| Molar volume | 9.53 cm³/mol |
| Crystal lattice of a simple substance | |
| Lattice structure | cubic body-centered |
| Lattice parameters | 3.160 Å |
| Debye temperature | 310K |
| Other characteristics | |
| Thermal conductivity | (300 K) 162.8 W/(mK) |
| CAS number | 7440-33-7 |
At 1580°C it is easily forged and drawn to a thin wire.
These advantages are created by the structure of the substance.
Refractory durable metal, light gray in color - tungsten
In air with a relative humidity of less than 60%, the metal’s resistance to corrosion is one hundred percent.
General information:
| 100 | General information | |
| 101 | Name | Tungsten |
| 102 | Former name | |
| 103 | Latin name | Wolframium |
| 104 | English name | Tungsten |
| 105 | Symbol | W |
| 106 | Atomic number (number in table) | 74 |
| 107 | Type | Metal |
| 108 | Group | Transition metal |
| 109 | Open | Carl Wilhelm Scheele, Sweden, 1781 (named), Juan José Elhujar Lubize and Fausto de Elhujar, Spain, 1783 |
| 110 | Opening year | 1783 |
| 111 | Appearance, etc. | Hard, refractory, shiny, silver-gray metal |
| 112 | Origin | Natural material |
| 113 | Modifications | |
| 114 | Allotropic modifications | 2 allotropic modifications: — α-tungsten with a cubic body-centered crystal lattice, - β-tungsten with a cubic crystal lattice, called phase A15 |
| 115 | Temperature and other conditions for the transition of allotropic modifications into each other | |
| 116 | Bose-Einstein condensate | |
| 117 | 2D materials | |
| 118 | Content in the atmosphere and air (by mass) | 0 % |
| 119 | Content in the earth's crust (by mass) | 0,00011 % |
| 120 | Content in seas and oceans (by mass) | 1,2·10-8 % |
| 121 | Content in the Universe and space (by mass) | 5,0·10-8 % |
| 122 | Abundance in the Sun (by mass) | 4,0·10-7 % |
| 123 | Content in meteorites (by mass) | 0,000012 % |
| 124 | Content in the human body (by weight) |
Receiving technology
Tungsten ores from different mining sites contain 0.3-2.5% metal oxide. Therefore, the industrial production of a product from ore begins at processing plants.
This is a multi-step process:
- Ore crushing.
- Grinding.
- Flotation.
- Burning.
The content of useful components increases to 60%:
- The purity of the concentrate is increased by breaking down impurities with sodium hydroxide and using the ion exchange extraction method.
- It is reduced to powder at 650-700°C in a hydrogen environment.
Refractoriness turned out to be a disadvantage that precluded classical fusion.
Solid forms are created using powder metallurgy:
- The powder is compressed.
- Sintering is carried out at 1250-1300°C in hydrogen.
- Affected by electricity.
- Heat to 3000°C, achieving monolithic sintering.
Tungsten powder
Additionally, the metal is cleaned by zone melting.
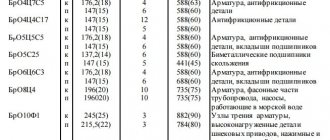
Nomenclature of metal grades
Based on tungsten or with its participation, metallurgists smelt products of dozens of names and brands.
Among the most common are pure tungsten (HF) and alloy with rhenium (HR).
The classification of tungsten grades is based on the composition of the additives:
| Brand name | Type of additive |
| VA | Aluminum + siliceous alkali |
| VM | Thorium + siliceous alkali |
| IN AND | Yttrium oxide |
| VT | Thorium oxide |
| VL | Lanthanum oxide |
How to use
The properties of tungsten identified the main consumer. This is metallurgy. It creates end products and sources for other industries.
Powdered tungsten is the basis or component of hard, heat-resistant, wear-resistant alloys and premium steel grades.
Metal, alloys
A wide range of products are created from refractory metals and alloys:
- Components and parts of aircraft and rocket engines.
- Elements of electric vacuum devices (picture tubes, incandescent filaments).
Tungsten filament - Vacuum furnace heaters.
- Electrodes for argon-arc welding. They do not melt and create a strong weld. Suitable for materials of any composition (non-ferrous metals, alloy steels, etc.).
- Containers for radioactive products. Here the advantages of the metal over lead turned out to be decisive.
- Surgical instruments.
The characteristics of the metal suited the defense complex: tank, torpedo armor, large-caliber shells, bullets. As well as super-high-speed rotors of gyroscopes that control the flight path of ballistic missiles.
Tungsten ingots
Connections
The range of applications for tungsten compounds is wide:
- Without ditelluride, it is impossible to convert heat into electricity.
- Carbide is the basis of alloys and composites for machining metals and non-metals. For miners, oil workers, gas workers - for drilling wells.
- Sulfide is a heat-resistant (up to 500°C) lubricant.
- Trioxide is a material for creating the electrolyte of fuel cells operating at elevated temperatures.
Tungsten compounds are purchased by manufacturers of varnishes, paints, and textiles.
Other forms
Isotope W184 is a component of alloys with uranium isotopes. They are used to make nuclear-fueled rocket engines.
A radionuclide of artificial origin (W185) is in demand as a radiation detector (including X-rays) in the nuclear segment of physics and medicine.
Use of tungsten
The use of tungsten is found in the following areas:
- Heat-resistant and wear-resistant alloys are based on the refractoriness of the substance. In industry, such chemical compounds are used with chromium and cobalt, which are otherwise called stellites. They are applied by surfacing to the wear area of parts in industrial vehicles.
- Heavy and contact alloys are mixtures of silver, copper, and tungsten. They can be called very effective contact components, which is why they are used for the production of working parts for switches, electrodes for spot welding, and also for the manufacture of switches.
- Tungsten is used as wire, forged products, and tape in radio engineering, in the creation of special electric lamps, and in X-ray engineering. It is this chemical element that is considered the best metal for the manufacture of spirals, as well as special filaments for incandescence.
- Tungsten rods and wire are needed to create special electric heaters for high-temperature furnaces. Tungsten heaters can operate in an inert gas atmosphere, in a vacuum, and also in hydrogen.