– (Wolframium), W – chemical element 6 (VIb) of the group of the periodic system of D.I. Mendeleev, atomic number 74, atomic mass 183.85. There are 33 known isotopes of tungsten: from 158W to 190W. Five isotopes have been discovered in nature, three of which are stable: 180W (share among natural isotopes 0.120%), 182W (26.498%), 186W (28.426%), and the other two are weakly radioactive: 183W (14.314%, T½ = 1.1 ·1017 years), 184W (30.642%, T½ = 3·1017 years). The electron shell configuration is [Xe]4f145d46s2. The most typical oxidation state is +6. Compounds with tungsten oxidation states +5, +4, +3, +2 and 0 are known.
Also on topic:
CHEMISTRY
Back in the 14th–16th centuries. miners and metallurgists in the Ore Mountains of Saxony noted that some ores interfered with the reduction process of tinstone (cassiterite mineral, SnO2) and led to slagging of the molten metal. In the professional language of that time, this process was characterized as follows: “These ores tear out the tin and devour it, like a wolf devours a sheep.” The miners gave this “annoying” breed the names “Wolfert” and “Wolfrahm”, which translated means “wolf foam” or “foam in the mouth of an angry wolf.” German chemist and metallurgist Georg Agricola in his fundamental work Twelve Books on Metals
(1556) gives the Latin name for this mineral - Spuma Lupi, or Lupus spuma, which is essentially a tracing of the German folk name.
In 1779, Peter Wulf investigated the mineral now called wolframite (FeWO4 x
MnWO4), and came to the conclusion that it must contain a previously unknown substance. In 1783 in Spain, the d'Elhuyar brothers (Juan Jose and Fausto D'Elhuyar de Suvisa) using nitric acid isolated from this mineral “acid earth” - a yellow precipitate of an oxide of an unknown metal, soluble in ammonia water. Oxides of iron and manganese were also found in the mineral. Juan and Fausto calcined the “earth” with charcoal and obtained a metal, which they proposed to call “tungsten”, and the mineral itself – “wolframite”. Thus, the Spanish chemists d'Elguiar were the first to publish information about the discovery of a new element.
Later it became known that for the first time tungsten oxide was discovered not in the “tin eater” - wolframite, but in another mineral.
In 1758, Swedish chemist and mineralogist Axel Fredrik Cronstedt discovered and described an unusually heavy mineral (CaWO4, later named scheelite), which he named Tung Sten, which means “heavy stone” in Swedish. Kronstedt was convinced that this mineral contained a new, not yet discovered, element.
In 1781, the great Swedish chemist Karl Scheele decomposed the “heavy stone” with nitric acid, discovering, in addition to the calcium salt, “yellow earth”, which was not similar to the white “molybdenum earth”, which he had first isolated three years earlier. Interestingly, one of the d'Elguiar brothers worked in his laboratory at that time. Scheele named the metal "tungsten", after the name of the mineral from which the yellow oxide was first isolated. So the same element got two names.
In 1821, von Leonhard proposed calling the mineral CaWO4 scheelite.
The name tungsten can be found in Lomonosov; Soloviev and Hess (1824) call it thistle, Dvigubsky (1824) - tungsten.
Back at the beginning of the 20th century. in France, Italy and the Anglo-Saxon countries, the element “tungsten” was designated as Tu (from tungsten). It was only in the middle of the last century that the modern symbol W was established.
History of discovery and study
The metal gets its name from the mineral wolframite. It began to be mined in the 16th century. Back then it was called "wolf's foam." Tungsten was often found in tin ores and interfered with the smelting of this metal. He converted it into a foam of slag.
The first scientific mention of the discovery of a new chemical element appeared in 1781. Then the famous chemist from Sweden Karl Scheele worked with the mineral scheelite. He treated it with nitric acid, during which he obtained a new chemical element with a yellow tint. He called it "heavy stone." Two years later, the Eluard brothers obtained a new metal from the Saxon mineral.
If we compare protection against ionizing radiation from lead or tungsten, the second type of metal wins. The finished protective layer will trap more particles with less weight.
Wolframite
Characteristics of alloys
The most important compound is tungsten carbide. It has a very high melting point - 2780 °C. It is used to make parts for electrical circuits, cutting tools, cermets and "cemented" carbide.
Metal-ceramics is a material made of ceramics and metal. Ceramics is a clay material. Metal ceramics are used where very high temperatures are exposed for a long time. For example, parts of a rocket or jet engine are made from it.
"Cemented" carbide is made by bonding tungsten carbide to another metal. The product is very durable and remains durable in high temperature environments. It is “cemented” carbides that are used for drilling tunnels. Tools made from this material can operate at speeds up to 100 times faster than similar tools made from steel (for example, drills made from this material can withstand higher temperatures than drills made from steel, and, therefore, the intensity of their use can be taller).
Extraction from ore and deposits
In nature, tungsten can be found as oxidized deposits. They are formed from the trioxide of this metal, which combines with calcium, manganese, and iron. Sometimes you can find copper, lead, thorium, and some rare earth elements in the composition.
Minerals saturated with tungsten are more often found in small inclusions in ground rocks. In this case, the average concentration of heavy metal is up to 2%.
The largest tungsten deposits are located in the USA, China, and Canada. The average global production per year is 50 thousand tons.
The critical temperature for this metal is 13610°C. When heated to such levels, it turns into gas.
Biological role of tungsten
limited. Its neighbor in the group, molybdenum, is indispensable in enzymes that ensure the fixation of atmospheric nitrogen. Previously, tungsten was used in biochemical studies only as an antagonist of molybdenum, i.e. replacing molybdenum with tungsten in the active site of the enzyme led to its deactivation. On the contrary, enzymes that are deactivated when replacing tungsten with molybdenum are found in thermophilic microorganisms. Among them are formate dehydrogenases, aldehyde ferredoxin oxidoreductases; formaldehyde ferredo-xyn oxidoreductase; acetylene hydratase; carboxylic acid reductase. The structures of some of these enzymes, such as aldehyde ferredoxin oxidoreductase, have now been determined.
Severe consequences of exposure to tungsten and its compounds on humans have not been identified. Long-term exposure to large doses of tungsten dust can cause pneumoconiosis, a disease caused by all heavy powders entering the lungs. The most common symptoms of this syndrome are cough, breathing problems, atopic asthma, changes in the lungs, the manifestation of which decreases after cessation of contact with metal.
Yuri Krutyakov
Industrial production
The production of tungsten by industrial enterprises begins with the extraction of ore and its delivery to production. The next stage is the separation of trioxide from the consumable material. It then goes through a reduction process to produce purified metal powder. The recovery procedure is carried out under the influence of hydrogen. In this case, the raw material is heated to 700°C. The finished powder is pressed and sintered at a temperature of 1300°C in a protective atmosphere of hydrogen.
Processing of tungsten raw materials.
The primary ore contains about 0.5% tungsten oxide. After flotation and separation of non-magnetic components, a rock containing about 70% WO3 remains. The enriched ore (and oxidized tungsten scrap) is then leached with sodium carbonate or hydroxide:
4FeWO4 + O2 + 4Na2CO3 = 4NaWO4 + 2Fe2O3 + 4CO2
6MnWO4 + O2 + 6Na2CO3 = 6Na2WO4 + 2Mn3O4 + 6CO2
WO3 + Na2CO3 = Na2WO4 + CO2
WO3 + 2NaOH = Na2WO4 + H2O
Na2WO4 + CaCl2 = 2NaCl + CaWO4Ї.
The resulting solution is freed from mechanical impurities and then processed. Initially, calcium tungstate is precipitated, followed by its decomposition with hydrochloric acid and dissolution of the resulting WO3 in aqueous ammonia. Sometimes purification of primary sodium tungstate is carried out using ion exchange resins. The final product of the process is ammonium paratungstate:
CaWO4 + 2HCl = H2WO4Ї + CaCl2
H2WO4 = WO3 + H2O
WO3 + 2NH3·
H2O(conc.) = (NH4)2WO4 + H2O
12(NH4)2WO4 + 14HCl(highly diluted) = (NH4)10H2W12O42 + 14NH4Cl + 6H2O
Another way to separate tungsten from enriched ore is by treating it with chlorine or hydrogen chloride. This method is based on the relatively low boiling point of tungsten chlorides and oxochlorides (300 ° C). The method is used to obtain especially pure tungsten.
Wolframite concentrate can be fused directly with coal or coke in an electric arc chamber. This produces ferrotungsten, which is used in the manufacture of alloys in the steel industry. Pure scheelite concentrate can also be added to the steel melt.
About 30% of global tungsten consumption is achieved through the processing of secondary raw materials. Contaminated tungsten carbide scrap, shavings, sawdust and tungsten powder residues are oxidized and converted into ammonium paratungstate. Scrap of high-speed steels is utilized in the production of the same steels (up to 60–70% of the total melt). Tungsten scrap from incandescent lamps, electrodes and chemical reagents is practically not recycled.
The main intermediate product in the production of tungsten is ammonium paratungstate (NH4)10W12O41·
5H2O. It is also the main transported tungsten compound. By calcining ammonium paratungstate, tungsten(VI) oxide is obtained, which is then treated with hydrogen at 700–1000 ° C to obtain metal tungsten powder. By sintering it with carbon powder at 900–2200° C (carburization process), tungsten carbide is obtained.
In 2002, the price of ammonium paratungstate, the main commercial tungsten compound, was about $9,000 per ton in terms of metal. Recently, there has been a downward trend in prices for tungsten products due to large supply from China and the countries of the former USSR.
In Russia, tungsten products are produced by: Skopinsky Hydrometallurgical Plant (Ryazan region, tungsten concentrate and anhydride), Vladikavkazsky (North Ossetia, tungsten powder and ingots), Nalchik Hydrometallurgical Plant (Kabardino-Balkaria, metal tungsten, tungsten carbide), Kirovgrad Hard Alloy Plant (Sverdlovsk region, tungsten carbide, tungsten powder), Elektrostal (Moscow region, ammonium paratungstate, tungsten carbide), Chelyabinsk Electrometallurgical Plant (ferrotungsten).
Stamps
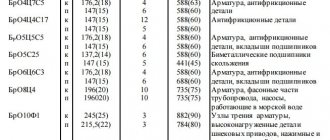
Tungsten grades:
- VR is a compound of tungsten and rhenium.
- VT, VI, VL - an additive of lanthanum, thorium, and yttrium oxide is added to the base.
- VRN is a metal without additives. The presence of a small amount of different impurities is allowed.
- VM - various additives are added to the base. The main ones are silica-alkaline, aluminum.
- MV is a compound of molybdenum and tungsten. Plasticity is maintained while strength is increased.
- HF is pure metal without impurities or additives.
- VA - combination of base with aluminum, silicon-alkaline additives.
Incandescent lamps have a sealed glass capsule for a reason. Since tungsten quickly oxidizes in open air, the capsule is filled with an inert gas.
Incandescent lamp
Manufacturing of posts
We have already found out what kind of metal tungsten is, and now we will find out in what range it is manufactured. Compact ingots - bars - are made from the powder compound. For this, only powder that has been reduced with hydrogen is used. They are made by pressing and sintering. The resulting ingots are quite strong, but fragile. In other words, they are difficult to forge. To improve this technological property, the bars are subjected to high-temperature treatment. A different assortment is made from this product.
Properties
To understand where it is best to use tungsten, you need to know the properties of this metal. Now enough information is known about this material to determine the scope of its application.
Chemical
Properties:
- The valency of a pure metal is 6. For compounds based on it, it can vary from 2 to 5.
- The molar mass of the chemical element is 183.84.
- The element has an orbit consisting of two tiers.
Tungsten is a reactive metal. It reacts with various substances to form complex, simple compounds. When heated, reactions proceed faster. To further speed up the reaction, water vapor can be added.
Physical
Properties:
- Color: grey.
- Transparency - none.
- There is a metallic sheen.
- Hardness - 7.5 (indicated according to the Mohs scale).
- Density - 19.3 g/cm3.
- Radioactivity - 0.
- Thermal conductivity - 173 W/(m K).
- Electrical conductivity - 55·10−9 Ohm m.
- The Brinell hardness index is 488 kgf/mm².
- Heat capacity - 134.4 J/(kg deg).
- Melting point - 3380 °C (the indicator depends on the amount of impurities).
- Electrical resistance - 55·10−9 Ohm·m (subject to a temperature regime of 20°C).
- Boiling point is about 5555 °C.
The metal is best forged when heated to 1600°C.
Heavy alloys are made based on tungsten. The total base content can reach 97%. Ready-made alloys are used for the manufacture of containers in which radioactive substances will be stored and transported. The main feature of the container is the ability to absorb part of the gamma radiation.
Application of tungsten.
The use of pure metal and tungsten-containing alloys is based mainly on their refractoriness, hardness and chemical resistance. Pure tungsten is used for the manufacture of filaments of electric incandescent lamps and cathode ray tubes, in the production of crucibles for the evaporation of metals, in the contacts of automobile ignition distributors, in the targets of X-ray tubes; as windings and heating elements of electric furnaces and as a structural material for space and other vehicles operated at high temperatures. High-speed steels (17.5–18.5% tungsten), stellite (cobalt-based with the addition of Cr, W, C), hastalloy (Ni-based stainless steel) and many other alloys contain tungsten. The basis for the production of tool and heat-resistant alloys is ferrotungsten (68–86% W, up to 7% Mo and iron), easily obtained by direct reduction of wolframite or scheelite con - a very hard alloy containing 80–87% tungsten, 6–15% cobalt, 5–7% carbon, indispensable in metal processing, mining and oil production.
Calcium and magnesium tungstates are widely used in fluorescent devices, and other tungsten salts are used in the chemical and tanning industries. Tungsten disulfide is a dry high-temperature lubricant, stable up to 500° C. Tungsten bronzes and other compounds of the element are used in the manufacture of paints. Many tungsten compounds are excellent catalysts.
For many years after its discovery, tungsten remained a laboratory rarity; only in 1847 did Oxland receive a patent for the production of sodium tungstate, tungstic acid and tungsten from cassiterite (tin stone). The second patent, obtained by Oxland in 1857, described the production of iron-tungsten alloys, which form the basis of modern high-speed steels.
In the middle of the 19th century. The first attempts were made to use tungsten in steel production, but for a long time it was not possible to introduce these developments into industry due to the high price of the metal. The increased demand for alloy and high-strength steels led to the launch of the production of high-speed steels at Bethlehem Steel. Samples of these alloys were first presented in 1900 at the World Exhibition in Paris.