The concept of metallurgy: general methods for producing metals
Metallurgy is the science of industrial methods for producing metals. There are ferrous and non-ferrous metallurgy.
Ferrous metallurgy is the production of iron and its alloys (steel, cast iron, etc.).
Non-ferrous metallurgy is the production of other metals and their alloys.
Metal alloys are widely used. The most common iron alloys are cast iron and steel.
Cast iron is an iron alloy containing 2-4 wt. % carbon, as well as silicon, manganese and small amounts of sulfur and phosphorus.
Steel is an iron alloy containing 0.3-2 wt. % carbon and small impurities of other elements.
Alloy steels are alloys of iron with chromium, nickel, manganese, cobalt, vanadium, titanium and other metals. The addition of metals gives steel additional properties. Thus, the addition of chromium gives the alloy strength, and the addition of nickel gives the steel ductility.
Main stages of metallurgical processes:
- Enrichment of natural ore (purification, removal of impurities)
- Production of metal or its alloy.
- Metal machining
Most metals occur in nature in the form of compounds. The most common metal in the earth's crust is aluminum. Then iron, calcium, sodium and other metals.
| Finding metals in nature | ||
| Active metals - in the form of salts | Medium activity metals - in the form of oxides and sulfides | Low-active metals - in the form of simple substances |
| Sodium chloride NaCl | ||
Active metals (alkali and alkaline earth) cannot be obtained from compounds using classical “chemical” methods. Such metals in the form of ions are very weak oxidizing agents, and in their simple form they are very strong reducing agents, so it is very difficult to reduce them from cations to simple substances. The more active the metal, the more difficult it is to obtain it in its pure form - because it tends to react with other substances.
Such metals can be obtained, as a rule, by electrolysis of molten salts , or by displacement of other metals from salts under harsh conditions.
Sodium is produced industrially by electrolysis of molten sodium chloride with calcium chloride additives:
2NaCl = 2Na + Cl2
Potassium is obtained by passing sodium vapor through a melt of potassium chloride at 800°C:
KCl + Na = K↑ + NaCl
Lithium can be obtained by electrolysis of molten lithium chloride in a mixture with KCl or BaCl2 (these salts serve to lower the melting point of the mixture):
2LiCl = 2Li + Cl2
Cesium can be obtained by heating a mixture of cesium chloride and specially prepared calcium:
Ca + 2CsCl = 2Cs + CaCl2
Magnesium is obtained by electrolysis of molten carnallite or magnesium chloride with additions of sodium chloride at 720–750°C:
MgCl2 → Mg + Cl2
Calcium is obtained by electrolysis of molten calcium chloride with calcium fluoride additives:
CaCl2 → Ca + Cl2
Barium is obtained from the oxide by reduction with aluminum in vacuum at 1200 °C:
4BaO+ 2Al = 3Ba + Ba(AlO2)2
Aluminum is produced by electrolysis of a solution of aluminum oxide Al2O3 in cryolite Na3AlF6:
2Al2O3 → 4Al + 3O2
Low-active and inactive metals are reduced from oxides with carbon, carbon monoxide (II) CO or a more active metal. Metal sulfides are first fired.
Concept of metallurgy
Metallurgy - the extraction of metals from ores - is one of the oldest types of human activity. Back in the second millennium BC. e. in Egypt they knew how to smelt iron from iron ore. The so-called Iron Age replaced the Bronze Age, which, in turn, came after the Stone Age.
Metals are obtained from ore minerals. For example, chalcopyrite or copper pyrite is a raw material for the production of iron, copper and sulfur ( Fig. 1 ). The chemical formula of the mineral is CuFeS2. Metals in other ores are in the form of oxides or salts of inorganic acids, chemically bonded cations.
Rice. 1. Chalcopyrite
The essence of the metallurgical process is the reduction of positive ions to free metal atoms. Carbon and its compounds, hydrogen, and metals are used as sources of electrons. During the reduction process, cations receive the missing electrons. The electron shells of the metal are restored. Process diagram:
Ме+n + ne- → Me, where
- Ме+n—metal in oxidized form;
- +n—oxidation state;
- ne- is the number of added electrons;
- Me is a metal in reduced form.
Pyrometallurgy
Pyrometallurgy
is a set of metallurgical processes occurring at high temperatures. This is a branch of metallurgy associated with the production and purification of metals and metal alloys at high temperatures, in contrast to hydrometallurgy, which involves low-temperature processes.
Description[ | ]
These are chemical processes occurring in metallurgical units at high (800-2000°C) temperatures. Therefore, pyrometallurgy is sometimes called “high temperature chemistry.”
Often chemical reactions are accompanied by a change in the aggregate state of the reacting substances: melting, sublimation, evaporation of the resulting metals or their compounds.
In such processes, interactions can occur between solid, liquid (melts) and gaseous phases in any combination.
Pyrometallurgical processes are the processes of agglomeration of metallurgical raw materials, smelting of charge materials, production of alloys, and refining of metals. In particular, this is roasting, blast furnace smelting, smelting in converters, arc and induction furnaces. Pyrometallurgy is the basis for the production of cast iron, steel, lead, copper, zinc, etc.
In pyrometallurgy, carbon reduction is often used - in cases where the metals being reduced do not form stable carbides, in addition to those mentioned above, such metals include germanium, cadmium, tin and others. In cases where stable carbides are formed by reduced metals, metallothermy is often used instead of reduction with carbon[1].
Pyrometallurgy is the main and most ancient field of metallurgy. From ancient times until the end of the 19th century, metal production was based almost exclusively on pyrometallurgical processes.
At the turn of the 19th and 20th centuries, another major branch of metallurgy, hydrometallurgy, acquired industrial importance.
However, pyrometallurgy continues to maintain a dominant position both in terms of production scale and variety of processes.
At the beginning of the 20th century, along with flame heating methods, different types of electric heating (arc, induction, etc.) began to be used in metallurgy; Around the same time, electrolysis of molten chemical compounds was introduced into industry (production of aluminum and other non-ferrous metals).
In the 2nd half of the 20th century, plasma melting of metals, zone melting, etc. became widespread. Metallurgical processes based on the use of electric current are classified as an independent field of pyrometallurgy - electrometallurgy.
Basic processes[ | ]
The main process of pyrometallurgy is ore smelting, which is carried out at such high temperatures that the products of chemical interaction melt, forming two liquid phases - metal or sulfide and slag. There are reduction and oxidation melts.
The defining process of reduction smelting is the reduction of metal oxides to ultimately produce a molten metal or its alloy with other elements. A typical reduction smelting process is the production of pig iron in blast furnaces. Reduction processes are also the main ones in the smelting of manganese, oxidized nickel, lead, and titanium ores.
The main reducing agents are carbon, carbon monoxide and hydrogen. Carbon monoxide is formed in the furnace itself when carbon is not completely burned; The main amount of hydrogen is obtained as a result of the decomposition of natural gas blown into the furnace.
A type of reduction smelting is the metallothermic production of metals, in which another metal with a greater affinity for oxygen is used as a reducing agent for a certain metal (Mn, Cr, V, etc.): Ca; Mg; Al and also Si. One of the advantages of metallothermic reduction is the production of metals that are not contaminated by carbon or hydrogen.
A typical oxidative ore smelting process is the processing of rich copper sulfide ores in shaft furnaces. During smelting, the bulk of the sulfur in sulfide minerals is oxidized, resulting in the release of a significant amount of heat. The main target product of smelting is the melt of FeS and Cu2S sulfides - matte.
Pig iron and ore smelting matte are essentially intermediate products that require additional processing. This treatment involves blowing the melts with air or pure oxygen, as a result of which the impurities contained in the alloys are oxidized and pass either into slag (SiO2; MnO; FeO, etc.) or into gas (CO; SO2). The process is called conversion.
A similar process to conversion is the fumming process—blowing slag melts with gas. Its difference from converting is that the metal melt is blown with an oxidizing gas, and when fumigating the slag, with a reducing gas.
And secondly, the products of oxidation of the metal melt - metal oxides - form a second liquid phase - slag, and the products of slag fuming - reduced volatile metals (or sulfides) in a vapor state are removed from the reaction space by a gas flow [2].
Literature[ | ]
Methods for obtaining metals
Depending on what reducing agent is used in the metallurgical process, they distinguish: pyro-, hydro, electro- and biometallurgy.
The most common methods for producing metals are pyrometallurgical and electrometallurgical. Most reduction reactions occur at high temperatures ( Fig. 2 ). Since the metallic bond has increased strength, the isolation of pure metals from natural compounds is carried out at high temperatures.
Rice. 2. Metallurgical production
Pyrometallurgical method
Pyrometallurgy is the extraction of metals from ores at high temperatures with the participation of reducing agents. Translated from Greek, “pyros” means “fiery.” Coke, carbon dioxide, and hydrogen are used as reducing agents. Active metals are used to obtain less active ones.
Pyrometallurgy is divided into
- carbothermy,
- hydrogenothermy,
- metallothermy.
Carbothermy: conversion of metal sulfide by firing into oxide and further reduction with coal to a pure state.
2ZnS + 3O2 = 2ZnO + 2 SO2
ZnO + C = CO + Zn
Ores consisting of iron oxides and sulfides are subjected to carbothermy. Reduction is carried out with coke or carbon dioxide (carbon monoxide). Iron alloys are produced - cast iron and steel. The first contains more carbon, as well as oxides of sulfur, phosphorus and silicon. Carbon reduces hardness and other qualities characteristic of metals.
Chemical reactions underlying iron smelting:
- C + O2 = CO2↑,
- CO2 + C ↔ 2CO↑,
- 3Fe2O3 + CO = 2Fe3O4+ CO2↑,
- Fe3O4 + CO = 3FeO + CO2↑,
- FeO + CO = Fe + CO2↑.
Steel is smelted in special furnaces - electric, converter, open-hearth ( Fig. 3 ). When blowing oxygen-enriched air, excess carbon burns out, its content decreases to 2% and below. This method is more economically applicable, because it is used to produce steel and cast iron, which are widely used in modern industry.
Rice. 3. Pyrometallurgy
By reducing coal you can obtain iron, copper, zinc, cadmium, germanium, tin, lead and other metals. Copper (Cu2O), tin (SnO2), manganese (MnO2) ores are used as raw materials.
| Scheme for obtaining iron and chromium | (Cr2Fe)O4 + 4C(coke) = Fe + 2Cr + 4CO↑ |
| The reaction underlying copper smelting | Cu2O + C (coke) = 2Cu + CO↑ |
| Tin production diagram | SnO2 + 2C (coke) = Sn + 2CO↑ |
| Manganese smelting process | MnO2 + C(coke) = Mn + CO2↑ |
| Lead production scheme | 2PbO + C → Pb + CO↑ |
Metals can be extracted from sulfide ores. First, firing is carried out, then the resulting oxide is reduced with coal. Schemes for roasting zinc blende and obtaining zinc:
- 2ZnS + 3O2 = 2ZnO + 2SO2↑;
- ZnO + C = Zn + CO↑.
Carbonates are also calcined with coal to produce oxides and subsequent reduction with coal. Schemes for firing siderite and reducing iron oxide:
- FeCO3 = FeO + CO2↑;
- FeO + C = Fe + CO↑.
Hydrogenothermy - production of metals by reduction with hydrogen
The advantage of this metallurgical method is the production of very pure metals. The reduction of copper from CuO oxide is an example of the reducing properties of hydrogen from a school course in inorganic chemistry. Reaction flow diagram (Fig. 4 ):
Rice. 4. Reduction of copper with hydrogen
Refractory metals molybdenum and tungsten are reduced from oxides with hydrogen.
Metallothermy
One metal is reduced by another, more chemically active one. This method is used to obtain metals from oxides and halides.
Depending on the nature of the reducing metal, aluminothermy or aluminothermy - reduction with aluminum and magnesiumthermy - reduction with magnesium are distinguished.
| Manganese production scheme | 3MnO2 + 4Al = 3Mn + 2Al2O3 |
| Chromium smelting process | Cr2O3 + 2Al → 2Cr + Al2O3 |
| Scheme for obtaining calcium | 4CaO+ 2Al= 2Ca+ (CaAl2)O4 |
Siliconothermy is the reduction of metals with silicon. The process proceeds according to the scheme: 2MgO + Si → 2Mg + SiO2.
3.2. Reduction of metals with coal
Pure metals can be obtained by reduction from oxides with carbon. In this case, only metal oxides located in the series of electrochemical activity after aluminum .
For example , iron is obtained by reduction from oxide with carbon:
2Fe2O3 + 6C → 2Fe + 6CO
ZnO + C → Zn + CO
Metal oxides, located in the series of electrochemical activity up to aluminum , react with coal to form metal carbides:
CaO + 3C → CaC2 + CO
Hydrometallurgical method
Hydrometallurgy is a method for producing noble, non-ferrous and rare metals. For example, copper oxide is first converted to sulfate using sulfuric acid. Copper is displaced from solution by iron. The following substitution reaction occurs: CuSO4 + Fe = Cu + FeSO4. Or copper is removed from solution by electrolysis. An electric current is passed through, Cu2+ ions are deposited on the cathode.
The advantage of the hydrometallurgical method is the ability to obtain metals from low-grade ores. Another advantage of the method is the reduction of gaseous emissions into the atmosphere. Large amounts of harmful gases and soot are released into the air during ore roasting and pyrometallurgy.
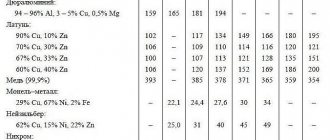
General characteristics of metal alloys
Now we will look at the general properties of metals and alloys by which they are characterized. They can very often be found in specialized literature.
| Characteristic | Decoding |
| Strength | The ability of an alloy to withstand mechanical loads and resist destruction. |
| Hardness | A property that determines the resistance of a material to attempts to introduce a part from another alloy or metal into its thickness. |
| Elasticity | The ability to restore its original shape after the application of significant mechanical force or load. |
| Plastic | On the contrary, this is a property that characterizes the possibility of changing shape and size under the influence of applied force, mechanical load. In addition, this also characterizes the ability of a part to maintain its newly acquired shape for a long time. |
| Viscosity | - the ability of a metal to resist rapidly increasing (shock) loads |
These are the qualities that characterize metal alloys. The table will help you understand them.
Advantages and disadvantages of magnesium alloys
They are quite soft, resist wear relatively well, but are not very impressive in their ductility. But they are distinguished by their excellent adaptability to forming at high temperatures, are perfectly suited for joining using all existing types of welding, and can also be connected by bolting, riveting and even gluing.
Alas, all these alloys are not particularly resistant to acids and alkalis. They are extremely negatively affected by prolonged exposure to sea water. However, magnesium alloys are surprisingly stable in air conditions, so many of their disadvantages can be neglected. If it is necessary to reliably protect such parts from corrosion, then chrome plating, anodizing or similar methods are used.
They can be clad with nickel, copper or chromium, after first immersing them in a melt of chemically pure zinc. With this treatment, their strength and abrasion resistance increase sharply. It must be recalled that magnesium is a fairly active metal from a chemical point of view, and therefore when working with it it is necessary to observe at least basic safety measures.
Thus, the production of metals and alloys is a key feature of modern industry. Every year people are inventing more and more ways to obtain new materials, so soon we will probably get absolutely incredible compounds that will combine the beneficial properties of several groups of materials and chemical elements at once.
Aluminum alloys
As we have already said, it is absolutely impossible to imagine modern industry without them. Judge for yourself: aluminum alloys are actively used in aviation, space, military, scientific and engineering and other industries. Without aluminum, it is impossible to imagine manufacturers of modern household and mobile appliances, since cases made of this metal are increasingly used by modern flagships of these industries.
Lead alloys
In general, non-ferrous metals and alloys are inseparably related concepts, since since ancient times people have been able to smelt multi-component materials, which they successfully used in military and peaceful affairs.
This especially applies to lead, from whose alloys the Romans made water pipes and sewerage systems. Of course, they did not know about the toxic properties of this metal, but they were impressed by the ease of its processing. The most commonly known solder at present is conventional solder, which is made from one part lead and two parts tin. As the name suggests, it is used for soldering parts. Used in radio engineering and other technical industries. Antimony and lead are used to make alloys that are used to make sheaths for various types of cables.
It has long been known that compounds of this metal with cadmium, bismuth or tin can melt at approximately 70 degrees Celsius. That is why today they are used to make various fuses in automatic fire extinguishing systems.
Oddly enough, lead has long been known to chefs and restaurateurs, as tableware and cutlery were often made from it. The alloy used for this is called pewter. It contains approximately 85–90% tin. The remaining 10-15% is occupied by lead (a standard alloy of two metals).
Technicians are also likely familiar with babbitts. These are also lead-based compounds that also contain tin, as well as arsenic and antimony. These alloys are very poisonous, but due to some special properties they are actively used in the bearing industry.
Copper alloys
Most often, this term refers to different types of brass. These are copper alloys that contain from 5 to 45% zinc. If its content ranges from 5-20%, then it is red brass (tompak). If the material already contains 20–36% Zn, then it is yellow brass.
These materials are ideal when it comes to producing and molding small parts. It is little known, but the alloy of copper and silicon is called silicon bronze and has great mechanical strength. The phosphorous variety is characterized by almost the same thing (5% tin and a certain amount of phosphorus are added to copper). As in the previous case, it is characterized by high strength and springy qualities, and therefore is ideal for the manufacture of membranes and various types of springs.
Magnesium alloys
They have extremely low weight and are also characterized by very impressive strength. In addition, these materials are excellent for the foundry industry, and the workpieces are perfectly amenable to turning and milling. Therefore, they are actively used in the production of missiles and aircraft turbines, instrument housings, car wheel rims, as well as some types of armor steel.
Some varieties of these alloys are distinguished by excellent viscous damping properties, and therefore they are used for the production of parts and structures that have to work under conditions of extremely high levels of vibration.
About light alloys
As we have already said, the properties of metals and alloys differ in that the latter in many cases have higher characteristics. This is especially noticeable in relation to modern industry. In recent years, it has required a huge amount of light alloys, which have increased mechanical strength, as well as resistance to adverse environmental factors and high temperatures.
Most often, aluminum, beryllium, and magnesium are used for their production. Compounds based on aluminum and magnesium are especially in demand, since the scope of their possible application is extremely wide.
Become
All iron compounds containing up to 2% carbon are called steels.
If the composition contains chromium, vanadium or molybdenum, then they are called alloyed. We encounter these materials constantly, daily and hourly. The number of steels today is such that listing them alone could fill a not-too-thin book. We have already said that the mechanical properties of metals and alloys are very different, but in the case of these materials, even different types of steel often have opposite qualities, which is why their areas of application differ greatly.
If the material contains less than 0.25% carbon, then it is used in some technical structures. If the steel contains more than 0.55% carbon, it is ideal for the production of a variety of high-quality cutting tools, including lathe cutters, drills and surgical supplies. But if we are talking about devices that are used for fast cutting, then only alloy steel is used for their production.