Steel has one drawback - it has magnetic properties, which are not always useful. Austenitic steel does not have this disadvantage. Such alloys have practically no magnetic properties, they do not rust, and withstand mechanical deformation well. Austenites are used for the production of radio equipment, turbines, and frost-resistant structures. What types of austenitic steels are there? How are various parts based on them welded?
Intergranular corrosion in austenitic stainless steels
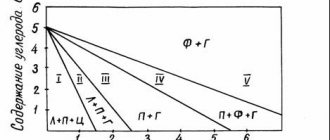
The tendency of steel to intergranular corrosion manifests itself as a result of the precipitation of carbide phases. Therefore, when assessing the corrosion properties of steel, the most important factor is the thermokinetic parameters of the formation of carbides in it.
The susceptibility to intergranular corrosion of hardened steel type 18-10 is determined, first of all, by the concentration of carbon in the solid solution. An increase in carbon content expands the temperature range of steel's susceptibility to intergranular corrosion.
Steel type 18-10, when held in the range of 750-800 ºС, becomes prone to intergranular corrosion:
- with a carbon content of 0.084% - already within 1 minute;
- with a carbon content of 0.054% - within 10 minutes;
- at a carbon content of 0.021 5 – after more than 100 minutes.
As the carbon content decreases, the temperature simultaneously decreases, which corresponds to the minimum duration of isothermal exposure before the onset of intergranular corrosion.
Stabilization of steel with titanium and niobium
When titanium and niobium, which promote the formation of carbides, are introduced into chromium-nickel steel type 18-10, the conditions for the precipitation of carbide phases change. At relatively low temperatures of 450-700 ºС, carbides of the Cr23C6 type are predominantly released, which give rise to a tendency to intergranular corrosion. At temperatures above 700 ºС, special carbides such as TiC or NbC are predominantly released. When only special carbides are isolated, there is no tendency to intergranular corrosion.
Application of alloys
Austenitic steels are used in the manufacture of devices operating at high temperatures, starting from 200 °C: steam generators, rotors, turbines and welding mechanisms. The disadvantage of using austenite in these mechanisms is the low strength of the metal. With prolonged contact of iron alloys with various hydroxides, additional cracks may form, which will lead to breakage of the working surfaces of devices. This drawback can be eliminated by adding additional chemical elements to the iron solution: vanadium and niobium. They form a carbide phase, which increases the strength of steel.
Nitrogen in austenitic stainless steels
Nitrogen, like carbon, has variable solubility in austenite. Nitrogen can form independent nitride phases during cooling and isothermal exposure or be part of carbides, replacing carbon in them. The effect of nitrogen on the susceptibility to intergranular corrosion of chromium-nickel austenitic steels is much weaker than that of carbon, and begins to appear only when its content is more than 0.10-0.15%. At the same time, the introduction of nitrogen increases the strength of chromium-nickel austenitic steel. Therefore, in practice, small additions of nitrogen are used in these steels.
General information
Austenitic steel is a special type of stainless steel. Austenitic steels contain iron, as well as various alloying components - nickel, manganese, nitrogen, aluminum, chromium, molybdenum.
Iron and alloying elements in steel form a cubic crystal lattice. This structure is called austenite. The crystal lattice determines a number of characteristic physical properties of austenite - preservation of hardness during heat treatment, almost complete absence of magnetic properties of the material, high chemical inertness.
For convenience, austenitic steels are divided into two conventional classes. The first category includes materials with a high nickel content. The second category includes materials with a high content of manganese and nitrogen, as well as a low nickel content.
The second materials have higher strength, but they cost an order of magnitude more. In addition, nickel-based austenite better withstands the effects of aggressive chemical environments (acids, alkalis, strong salts, radioactive substances).
Various techniques, things, and equipment are made from austenite steel. This could be metering devices, cutlery, metal beams, turbines, structural elements, automotive parts, special equipment for the needs of the chemical industry, and so on.
Another major application of austenite is the manufacture of radio equipment. The absence of magnetic properties in this case is beneficial - ordinary steel alloys can introduce certain distortions into the radio signal, while austenite will transmit the signal without delays, losses, or distortions.
Effect of chromium content
With increasing chromium concentration, the solubility of carbon in chromium-nickel austenite decreases, which facilitates the precipitation of the carbide phase in it. This, in particular, is confirmed by a decrease in the impact toughness of steel with an increase in chromium content, which is associated with the formation of a carbide network along the grain boundaries.
At the same time, an increase in the concentration of chromium in austenite leads to a significant decrease in the susceptibility of steel to intergranular corrosion. This is explained by the fact that chromium significantly increases the corrosion resistance of steel. A higher concentration of chromium in steel results in a lower degree of depletion of grain boundaries when carbides are precipitated there.
Delta Ferrite in Chromium Molybdenum Austenitic Steel
The presence of delta ferrite in the structure of austenitic chromium-nickel steel type 18-10 has a negative effect on its manufacturability during hot plastic deformation - rolling, piercing, forging, stamping.
The amount of ferrite in steel is strictly limited by the ratio of chromium and nickel in it, as well as by technological means. The group of steels most prone to the formation of delta ferrite is the X18N9T type (see also Stainless steels). When these steels are heated to 1200 ºС, the structure can contain up to 40-45% delta ferrite. The most stable are steels of the X18N11 and X18N12 types, which, when heated at high temperatures, retain an almost purely austenitic structure.
Checking stainless fasteners using a magnet
A characteristic of products made of austenitic steel may be their magnetic property. The attraction of a magnet to products A1-A5 indicates the low quality of the material. This is also defined by the international standard ISO 3506 (GOST R ISO 3506 in the Russian Federation). According to this standard, all fasteners using austenitic stainless steels are non-magnetic under normal conditions, but cold working or other mechanical processing may result in certain magnetic characteristics. The property of each material is the ability to magnetize, which can also be used for stainless steels. As is known, only vacuum is considered completely non-magnetic.
Martensite in chromium-nickel austenitic steels
Within the grade composition in steels of the X18N10 type, chromium, nickel, carbon and nitrogen contribute to a decrease in the temperature of the martensitic transformation, which is caused by cooling or plastic deformation.
The influence of titanium and niobium can be twofold. Being in solid solution, both elements increase the stability of austenite with respect to martensitic transformation. If titanium and niobium are bound into carbonitrides, then they can slightly increase the temperature of the martensitic transformation. This happens because austenite in this case is depleted of carbon and nitrogen and becomes less stable. Carbon and nitrogen are strong austenite stabilizers.
Heat treatment of chromium-nickel austenitic steels
For chromium-nickel austenitic steels, two types of heat treatment are possible:
- hardening and
- stabilizing annealing.
Heat treatment parameters differ for unstabilized steels and steels stabilized with titanium or niobium.
Hardening is an effective means of preventing intergranular corrosion and giving steel an optimal combination of mechanical and corrosion properties.
Stabilizing annealing of hardened steel converts chromium carbides:
- to a state that is not dangerous for intergranular corrosion for non-stabilized steels;
- into special carbides for stabilized steels.
Hardening of austenitic chromium-nickel steels
In steels without titanium and niobium additives, hardening means heating above the dissolution temperature of chromium carbides and fairly rapid cooling, which fixes a homogeneous gamma solution. The heating temperature for quenching increases with increasing carbon content. Therefore, low-carbon steels are hardened at lower temperatures than high-carbon steels. In general, the heating temperature range is from 900 to 1100 ºС.
The duration of exposure of steel at the hardening temperature is quite short. For example, for sheet material, the total heating and holding time when heated to 1000-1050 ºС is usually selected at the rate of 1-3 minutes per 1 mm of thickness.
Cooling from the quenching temperature must be rapid. For unstabilized steels with a carbon content of more than 0.03%, cooling in water is used. Steels with a lower carbon content and small cross-sections of the product are cooled in air.
Heat treatment features
Despite the fact that this material has increased strength characteristics, it is very difficult to process metal. Typically, to improve the quality of the workpiece, one of the following methods is used:
- Annealing. This process involves heating to high temperatures (changing the crystal lattice) followed by holding for several hours. After this, cooling occurs in one of the following ways: in oil, water, or in air at room conditions. This helps to reduce the hardness of austenitic steels.
- Double hardening. Repeated heating procedure increases the heat resistance of the material. Additionally, aging is often used.
Austenite is a very commonly used alloy. To understand the topic in more detail, watch the video:
Stabilizing annealing of austenitic chromium-nickel steels
In unstabilized steels, annealing is carried out in the temperature range between the heating temperature for hardening and the maximum temperature for the manifestation of intergranular corrosion. The value of this interval primarily depends on the chromium content in the steel and increases with increasing its concentration.
In stabilized steels, annealing is carried out to transfer carbon from chromium carbides to special titanium and niobium carbides. In this case, the released chromium goes to increase the corrosion resistance of steel. The annealing temperature is usually 850-950 ºС.
Resistance of austenitic chromium-nickel steels to acids
The ability to passivate provides chromium-nickel austenitic steels with fairly high resistance to nitric acid. Steels 12Х18Н10Т, 12Х18Н12Б and 02Х18Н11 have the first resistance rating:
- in 65% nitric acid at temperatures up to 85 ºС;
- in 80% nitric acid at temperatures up to 65 ºС;
- 100% sulfuric acid at temperatures up to 65 ºС;
- in mixtures of nitric and sulfuric acids: (25% + 70%) and 10% + 60%) at temperatures up to 70 ºС;
- in 40% phosphoric acid at 100 ºС.
Austenitic chromium-nickel steels also have high resistance to solutions of organic acids - acetic, citric and formic, as well as alkalis KOH and NaOH.
Welding technologies
To minimize the occurrence of defects in the further operation of chromium-nickel steels, it is necessary to correctly select the optimal method for welding austenitic steel.
The main methods of welding austenitic steel:
- manual arc;
- electroslag;
- in an atmosphere of protective gases.
Manual arc welding
Manual arc welding is a fairly maneuverable method. This welding occurs in such a way that the chemical composition remains unchanged at different spatial positions and possible positions of the joints.
Optimal recommendations for manual arc welding:
- thread seams using electrodes with a cross-section of 3 millimeters;
- Calcinate the welding electrodes for 60-90 minutes at a temperature from 250 oC to 400 oC (this must be done before starting welding). This prevents the formation of pores in the connecting seam.
Suitable electrodes are used with direct current and always with reverse polarity. At maximum current, welding is performed in the bottom position. And if work is required in a vertical or ceiling position, you need to take 10-30% less current.
Electroslag welding
The technology of performing electroslag welding work itself minimizes the possibility of hot cracks forming.
Advantages of this welding technique:
- No significant deformations in the corner and joint areas.
- Slow speed of movement of heating equipment.
- Soft crystallization of the weld pool.
Electroslag welding diagram
For this type of welding, electrodes in the form of plates with a thickness of 6 to 20 mm or wire with a thickness of 3 mm are used.
Welding under shielding gases
Welding in an atmosphere of protective gases allows you to perform work on products of various thicknesses. In this technology, active and inert gases work positively. Due to the variety of protective gases, the welder independently selects the conditions for introducing the required amount of heat into the metal and can change the efficiency of the electric arc.
This type of work is characterized by the use of tungsten or consumable electrodes. They are great for 5-7 mm products.
Welding is performed with a pulsed or burning arc. It is more optimal to use the first type, since during pulsed operation the distortion of the edge configuration is reduced, and the length of the heat-affected zone is also reduced.
Tungsten electrodes can be used with or without filler material. This depends on the thickness of the joint being connected and the design of the part.
For active gases and mixtures of gases, consumable electrodes are used. Rods of this type contribute to high quality work when used in pulsed arc welding. This technique is performed in a mixture of oxygen, carbon dioxide and argon, as well as in pure argon.