Austenitic
The most common type of steel. The structure includes the addition of nickel, manganese and nitrogen. These steels have excellent corrosion resistance and after annealing they become non-magnetic. They are excellent for welding and extremely hygienic due to their properties, they cannot be hardened by heat treatment but have an excellent level of performance at low temperatures. Corrosion resistance can be increased by adding chromium, molybdenum and nitrogen. Standard austenitic steels are susceptible to corrosion.
Methods for obtaining copper
In nature, copper exists in compounds and in the form of nuggets. The compounds are represented by oxides, bicarbonates, sulfur and carbon dioxide complexes, as well as sulfide ores. The most common ores are copper pyrite and copper luster. The copper content in them is 1-2%. 90% of primary copper is mined using the pyrometallurgical method and 10% using the hydrometallurgical method.
Pyrometallurgical method
The pyrometallurgical method includes the following processes: enrichment and roasting, smelting for matte, purging in a converter, electrolytic refining. Copper ores are enriched by flotation and oxidative roasting. The essence of the flotation method is as follows: copper particles suspended in an aqueous medium adhere to the surface of air bubbles and rise to the surface. The method allows you to obtain copper powder concentrate, which contains 10-35% copper.
Copper ores and concentrates with a significant sulfur content are subject to oxidative roasting. When heated in the presence of oxygen, sulfides are oxidized, and the amount of sulfur is reduced by almost half. Poor concentrates containing 8-25% copper are roasted. Rich concentrates containing 25-35% copper are melted without resorting to roasting.
The next stage of the pyrometallurgical method for producing copper is smelting for matte. If lump copper ore with a large amount of sulfur is used as a raw material, then smelting is carried out in shaft furnaces. And for powdered flotation concentrate, reverberatory furnaces are used. Melting occurs at a temperature of 1450 °C.
In horizontal converters with side blowing, the copper matte is blown with compressed air in order for the oxidation of sulfides and ferrum to occur. Next, the resulting oxides are converted into slag, and sulfur into oxide. The converter produces blister copper, which contains 98.4-99.4% copper, iron, sulfur, as well as small amounts of nickel, tin, silver and gold.
Blister copper is subject to fire and then electrolytic refining. Impurities are removed with gases and converted into slag. As a result of fire refining, copper is formed with a purity of up to 99.5%. And after electrolytic refining, the purity is 99.95%.
Hydrometallurgical method
The hydrometallurgical method involves leaching copper with a weak solution of sulfuric acid, and then separating copper metal directly from the solution. This method is used for processing low-grade ores and does not allow for the associated extraction of precious metals along with copper.
Copper Applications
Due to their valuable qualities, copper and copper alloys are used in the electrical and electrical engineering industries, in radio electronics and instrument making. There are alloys of copper with metals such as zinc, tin, aluminum, nickel, titanium, silver, and gold. Less commonly used are alloys with non-metals: phosphorus, sulfur, oxygen. There are two groups of copper alloys: brass (alloys with zinc) and bronze (alloys with other elements).
Copper is highly environmentally friendly, which allows its use in the construction of residential buildings. For example, a copper roof, due to its anti-corrosion properties, can last more than a hundred years without special care or painting.
Copper in alloys with gold is used in jewelry. This alloy increases the strength of the product, increases resistance to deformation and abrasion.
Copper compounds are characterized by high biological activity. In plants, copper takes part in the synthesis of chlorophyll. Therefore, it can be seen in the composition of mineral fertilizers. A lack of copper in the human body can cause deterioration in blood composition. It is found in many food products. For example, this metal is found in milk. However, it is important to remember that excess copper compounds can cause poisoning. This is why you should not cook food in copper cookware. During boiling, large amounts of copper can leach into food. If the dishes inside are covered with a layer of tin, then there is no danger of poisoning.
In medicine, copper is used as an antiseptic and astringent. It is a component of eye drops for conjunctivitis and solutions for burns.
Where are copper products most often used?
The main area of application of aluminum and copper is known, perhaps, to everyone. They are used to make a variety of cables, including power cables. This is facilitated by the low resistance of aluminum and cuprum and their special magnetic capabilities. In the windings of electric drives and in transformers (power), copper wires are widely used, which are characterized by the unique purity of copper, which is the raw material for their production. If you add only 0.02 percent aluminum to such pure raw materials, the electrical conductivity of the product will decrease by 8–10 percent.
Cu, which has high density and strength, as well as low weight, is perfectly amenable to machining. This allows us to produce excellent copper pipes that demonstrate their high performance characteristics in gas, heating, and water supply systems. In many European countries, copper pipes are used in the vast majority of cases for the arrangement of internal utility networks of residential and administrative buildings.
We have said a lot about the electrical conductivity of aluminum and copper. Let's not forget about the excellent thermal conductivity of the latter. This characteristic makes it possible to use copper in the following structures:
- in heat pipes;
- in coolers of personal computers;
- in heating systems and air cooling systems;
- in heat exchangers and many other devices that remove heat.
The density and light weight of copper materials and alloys have also led to their widespread use in architecture.
How to identify metal: types of tests, use of chemistry
Probably everyone had to hold in their hands a piece of jewelry or another object, obviously metal.
But how can you determine what metal is used in production? It could be a precious material or a counterfeit, or even a trinket with no claims to value. Expertise from specialists will give you the exact answer, but it is not free.
But there are methods for approximately determining the type of metal at home. They were used a long time ago, but they have not lost their relevance in our time.
Magnet check
Bringing a magnet close to the item being tested is a good way to perform initial testing. By the reaction of the magnet you can determine which group the metal belongs to:
Of course, such a check will not allow us to accurately determine the material from which the item is made. After all, a non-magnetic metal may not be in its pure form, but in the form of an alloy with a ferromagnet. But it can confirm or refute the assumption. For example, if it is checked whether it is gold or not, but the item is clearly magnetic, then it can be argued that it is a fake.
When checking jewelry, you should take into account that, in addition to precious metals, they may contain clasps, built-in springs, made of another material. You need to check the metal itself.
Heat check
You can also determine the group of a metal by how it conducts heat. It is known that the thermal conductivity of silver is very high. It is almost five times higher than that of iron or platinum. Slightly worse for gold, copper and aluminum. Platinum transfers heat even weaker than iron.
If you immerse the metal in hot water for 15–20 seconds, then based on its temperature, determined by touch, you can draw some conclusions.
In this way it is easy to distinguish platinum from silver. But it’s not possible to compare silver or aluminum alloy.
Iodine test
You can check the authenticity of the metal using an iodine solution purchased at a pharmacy. A drop of iodine is applied to the surface and left for several seconds. Iodine will not harm noble metals - gold, platinum, silver. If the color of a drop of iodine does not change, and after removing it with a napkin, no traces or stains remain, this indicates the authenticity of the metal. If darkening is visible at the place of the drop, then this is a low-quality alloy or an outright fake.
Vinegar test
Household vinegar solution also does not affect precious metals. And it is dangerous for counterfeits. But, unlike the iodine test, acetic acid takes time. To wait for the result, you need to immerse the metal being tested in a container with vinegar for 15–30 minutes. The absence of traces of interaction between metal and vinegar is a sign of nobility.
If, in addition to metal, the product contains precious or semi-precious stones, then it is better not to check them this way; vinegar can ruin them. This is especially true for pearls.
Application of chemicals
Testing with active chemical reagents should be left as a last resort. If handled improperly, they will damage even genuine precious metal. And they can be dangerous for the health of the inspector.
Alkali metals are the MOST DANGEROUS and Active Elements! (December 2019).
Magnets are materials that create magnetic fields that attract certain metals. Every magnet has a north and south pole. Reverse poles attract while poles repel.
While most magnets are made from metals and metal alloys, scientists have developed ways to create magnets from composite materials such as magnetic polymers.
What creates magnetism?
Magnetism in metals is created by the uneven distribution of electrons in the atoms of certain metallic elements.
The uneven rotation and motion caused by this uneven distribution of electrons shifts the charge within the atom back and forth, creating magnetic dipoles.
When magnetic dipoles align, they create a magnetic domain, a localized magnetic region with north and south poles.
In non-magnetic materials, magnetic domains collide in different directions, canceling each other out. Whereas in magnetized materials, most of these domains are aligned, pointing in the same direction, which creates a magnetic field. The more areas that line up with each other, the stronger the magnetic force.
Magnet types:
- Permanent magnets (also known as hard magnets) are those that constantly produce a magnetic field. This magnetic field is caused by ferromagnetism and is the strongest form of magnetism.
- Temporary magnets (also known as soft magnets) are only magnetic in the presence of a magnetic field.
- Electromagnets require electric current to pass through their coil wires to create a magnetic field.
Development of magnets:
Greek, Indian and Chinese writers documented basic knowledge of magnetism more than 2,000 years ago. Much of this understanding was based on observations of the effects of magnesium (the naturally occurring magnetic mineral of iron) on iron.
Early research into magnetism dates back to the 16th century, but the development of modern high-strength magnets did not occur until the 20th century.
Before 1940, permanent magnets were used only in basic applications such as compasses and electrical generators called magnetos. The development of aluminum and nickel-cobalt (Alnico) magnets has allowed permanent magnets to replace electromagnets in motors, generators and loudspeakers.
The development of samarium-cobalt (SmCo) magnets in the 1970s created magnets with twice the magnetic energy density of any previously available magnet. Smaller, more powerful magnets contributed to the development of many electronic devices as we know them.
By the early 1980s, further research into the magnetic properties of rare earth elements led to the discovery of neodymium-iron-boron (NdFeB) magnets. NdFeB magnets again led to a doubling of magnetic energy over SmCo magnets.
Rare earth magnets are now used in everything from wristwatches and iPads to hybrid car engines and wind turbines.
Magnetism and temperature:
Metals and other materials have different magnetic phases, depending on the temperature of the environment in which they are located. As a result, a metal can exhibit more than one form of magnetism.
Iron, for example, loses its magnetism, becoming paramagnetic when heated above 1418 °F (770 °C).
The temperature at which a metal loses its magnetic force is called its Curie temperature.
Iron, cobalt and nickel are the only elements that, in metallic form, have Curie temperatures above room temperature. Thus, all magnetic materials must contain one of these elements.
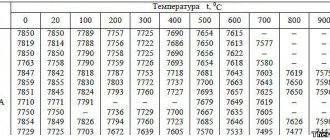
Common ferromagnetic metals and their curie temperatures:
| Substance | Curie temperature |
| Iron (Fe) | 1418 °F (770 °C) |
| Cobalt (Co) | 2066 °F (1130 °C) |
| Nickel (Ni) | 676.4°F (358°C) |
| Gadolinium | 66°F (19°C) |
| Dysprosium | -301. 27°F (-185.15°C) |
Sources: How Stuff Works, Inc. How do magnets work? //science. How it works. com/magnet1. HTM Wikipedia. Curie temperature. // ru. Wikipedia. org/wiki/Curie_temperature
Diamagnets
In diamagnetic materials, only the spin moments are compensated. If you bring such a substance to a magnet, then the motion of electrons under the influence of an external magnetic field will be added to the orbital magnetic moment. This will create an additional current, the magnetic field of which will be directed against the external magnetic field, so the diamagnetic materials will be repelled from the magnet.
Therefore, if we speak scientifically about which metals are not magnetic to a magnet, then these are diamagnetic materials, their list includes lithium and beryllium.
Non-magnetic stainless steels
Stainless steels that are not magnetic include chromium-nickel and chromium-manganese-nickel. They are usually divided into several groups.
Austenitic
The most popular brand of such stainless steels, which occupy a leading place among non-magnetic steel alloys, is 08Х18Н10 (international analogue according to AISI 304 classification). Steels of this type, which also include 08Х18Н10, 08Х18Н10Т, 12Х18Н10Т, 10Х17Н13М2Т, are actively used in the production of equipment for the food industry; kitchenware and cutlery; plumbing equipment; containers for food liquids; refrigeration equipment elements; containers for food products; medical supplies, etc.
Composition and application of austenitic steels
The great advantages of such stainless steel, which does not have magnetic properties, are its high corrosion resistance, demonstrated in many aggressive environments, and manufacturability.
Austenitic-ferritic
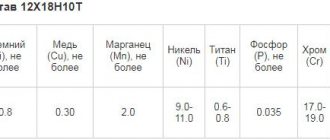
Steels of this group, the most popular grades of which are 08Х22Н6Т, 08Х21Н6М2Т and 12Х21Н5Т, are distinguished by a high chromium content and a low nickel content. To give such a stainless steel the required characteristics (an optimal combination of high strength and good ductility, resistance to intergranular corrosion and stress-corrosion cracking), elements such as copper, molybdenum, titanium or niobium are introduced into its chemical composition.
Chemical composition of some industrial grades of austenitic-ferritic steels (click to enlarge)
In addition to the above, stainless steels that are not magnetic include alloys with an austenitic-martensitic and austenitic-carbide structure.
Duplex
This type has a structure that is approximately 50% ferritic and 50% austenitic steel. This gives higher strength than the others and has increased resistance to chloride. Superduplex steels have increased strength and resistance to all types of corrosion compared to standard austenitic steels. They are weldable, but require care in the selection of welding materials and heat input. They have moderate formability, are magnetic, but not as magnetic as ferritic, martensitic and PH classes due to the 50% austenitic phase.
Paramagnets and ferromagnets
Let's consider the option when each atom of a substance has its own magnetic field. These fields are multidirectional and compensate each other. If you place a magnet next to such a substance, the fields will be oriented in one direction. The substance will have a magnetic field, a positive and a negative pole. Then the substance will be attracted to the magnet and can itself become magnetized, that is, it will attract other metal objects. For example, you can magnetize steel clips at home. Each one will have a negative and a positive pole, and you can even hang a whole chain of paper clips on a magnet. Such substances are called paramagnetic.
Ferromagnets are a small group of substances that are attracted to magnets and are easily magnetized even in a weak field.