In many areas of industry, construction and repair, tools, fasteners and hardware made of stainless steel are used. But despite the fact that this material has increased resistance to corrosion, rust may still appear in some cases. To prevent this, it is necessary to take additional measures - chemical passaging of products.
What is passivation?
The passivation process allows stainless steel to return to its original properties, further protecting it from the effects of many external factors. This is a special chemical treatment of metal products, after which a special protective coating is formed on their surface. When interacting with concentrated acids, an inconspicuous film appears on stainless steel. This process is called passivation.
This method is used both for additional processing during the production of products and for restoring the basic properties of stainless steel parts.
What oxidizing agents are required for passivation?
The main condition for passivation of stainless steel is that passivation does not destroy the base metal. Therefore, the oxidizing agent must be “soft”, with a relatively low pH. Under such conditions, a protective passive film forms spontaneously. It is better to use citric acid as such substances, since organic acids work more gently than mineral ones, and they do not require special preparation.
Is it possible to do without passivation? Stainless steel has corrosion-resistant properties due to its chromium content, but is not completely impervious to corrosion. Oxidizing in the presence of citric acid, chromium forms a moisture-resistant surface film.
Corrosion of stainless steel can be caused by:
- Foreign material in the work environment;
- Sulfides, which are often added to stainless steel to improve its machinability;
- Iron particles from cutting tools moving are transferred to the surface of parts during machining.
Why is this necessary?
A stainless steel sheet has a very thin oxide film on its surface. It is this that prevents the formation of rust on parts, fasteners, and hardware made from this material. But the slightest violation of the integrity of this coating leads to the fact that the main anti-corrosion properties of stainless steel are lost. The causes of damage to the oxide film can be very different:
when the material comes into contact with chlorine; when steel interacts with sea water; in case of mechanical or physical damage, including scratches and minor dents.
Therefore, it is important to comply with the operating conditions that are regulated by the manufacturing plants of certain products (cutlery, fasteners, hardware, working tools, solid sheets, etc.). It is prohibited to use detergents containing chlorine and other aggressive chemicals.
But the greatest damage to the oxide film is caused by welding. This is especially harmful in the case of pipe welding. In such a situation, the protective surface is destroyed along the entire seam. Steel passivation is used to restore surfaces and protect products from rust. But here the composition of the stainless steel plays an equally important role.
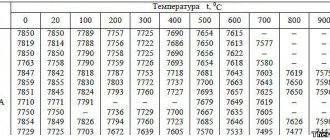
Reasons for metal stability
The corrosion process is characterized by the fact that, gradually oxidizing under the influence of negative factors, the surface of stainless steel is destroyed. If no measures are taken, the destruction will affect its deeper layers.
Table of stability of metals in different environments
Passivation of the metal avoids this problem. The surface of the product is covered with a protective oxide film, and special additives included in the treatment solution improve the properties of the stainless steel. The new material is not damaged.
In industrial conditions, it is possible to obtain a layer of corrosion protection that is ideal in thickness and uniformity. If the conditions in which the product will be used are not too aggressive, then it does not need additional processing. It is important to remember that mechanical damage to steel gives impetus to corrosion processes.
Stainless steel classification
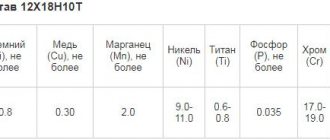
The anti-corrosion properties of stainless steel directly depend on its composition. Based on this, this steel is marked. The classification allows you to distinguish each type of stainless metal by flexibility, hardness, and degree of anti-corrosion protection. Depending on the composition and purpose, they are distinguished:
martensitic steels. Knives (including those for the food industry) and turbines are usually made from them. This steel, having a large percentage of chromium in its content, is very hard; ferritic materials. The amount of chromium in such steel exceeds the previous value by 3-4%. This material has high resistance to phosphoric acid, ammonium nitrate and nitric acid; austenitic steels. This type of stainless steel is very ductile. It is often used in mechanical engineering; duplex or ferro-austenitic metals. These are very durable, but at the same time flexible stainless materials.
Based on the composition of the stainless steel, you can determine whether there is a need for additional processing of the products or not. The likelihood of corrosion on the surface of elements made from this type of steel also depends on this.
Essence of the process
Passivation is not an electrolytic finishing operation that increases the corrosion resistance of stainless steels. The passivation process typically uses dilute nitric or citric acid to promote the formation of an inert protective oxide layer. It is more inert to air, so it slows down subsequent corrosion.
The acid chemically removes—dissolves—free iron from the stainless steel surface, replacing it with a thin surface film of less reactive oxides. Since any stainless steel contains a large amount of chromium, passivation results in the formation of chromium oxide, which has an increased thickness. The surface is passivated and rust protection is improved. At the same time, surface contaminants are removed.
Technology and methods
There are various methods for processing stainless steel. But there are two main methods of steel passivation:
Etching with chemical acids (concentrates) in certain areas. This technology is often used for processing welds, but is also allowed in other cases. This process has different processing sequence options. They differ both in the composition of chemicals and in the time of work. The most common method in this case is electrolytic etching. This technology consists of placing a stainless steel product in a specially prepared bath consisting of concentrated acids. An electric current (alternating or direct) is passed through this composition. The metal plays the role of either a cathode or anode. The supplied current has a mechanical effect on the steel, resulting in the release of hydrogen or oxygen gas. This helps to separate the oxide film on the surface of the product. Etching with ready-made acid mixtures. They can be made in the form of pastes, gels, sprays, concentrates. This method is the most convenient.
Regardless of which method is used to passivate stainless steel, it is important to follow the sequence of work.
Electrochemical passivation of stainless steel seams
One of the ways to passivate stainless steel welds is the electrochemical method
. According to the technology, the heat-affected zone is exposed to electric current and specially developed electrolytes for cleaning, etching, passivation and polishing of stainless steel. When current flows through a solution, changes occur in the chemical composition of the substances involved in the reaction.
When removing tarnish from stainless steel seams, the surface does not deteriorate, this means that if you use “mirror” steel, then after processing you will not see dull, dull spots in the heat-affected area, which can remain when using nitrogen-containing etching pastes. Also, during electrochemical passivation of stainless steel welds, nothing happens to either the matte or polished surface. Moreover, after processing the seams using this technology, the alloying (passive) layer of stainless steel is completely restored, which during further operation protects the steel surface from corrosion.
Apparatus for passivation of welds
Stainless steel welds can be cleaned and passivated using special equipment, one example of which is the SteelGuard series welders.
. These units are designed to perform cleaning of stainless steel welds, including the final functions of passivation and polishing of stainless steel seams.
Equipment such as the SteelGuard 685 electrochemical seam cleaner allows you to control the required current strength, so you can work effectively on any type of surface without fear of damaging the metal when touching a bare electrode, which previously left an irreparable mark and spoiled the surface of stainless steel.
Our company’s case study of replacing the chemical method of passivation of welds with an electrochemical method in food production can be seen in the article “Apparatus for passivation of welds in food production PTK NIKA.”
Stages of chemical passivation
In the process of forming a homogeneous inert film on the surface of stainless steel products, it is important to take into account the characteristics of the steel composition and the degree of damage to the protective coating. Chemical passivation today is an integral part of working with stainless materials. This allows you to extend their service life, get rid of rust and damage, and prevent the formation of corrosion. During passivation work, the following sequence of steps should be followed:
First, the materials are cleaned from contaminants. Grease stains, rust and other deposits are removed. With chemical acid etching technology, the product is immersed in a bath with a mixture of hydrochloric acid and sulfuric acid. At temperatures from 60 to 80 degrees, the steel is kept here for 20-40 minutes. If the method of etching with ready-made mixtures of acids is used, then special concentrated compositions (pastes, gels, sprays) are used for cleaning, which are applied to the surface of the steel manually. The chemical is left for approximately 30 minutes. Then the products are thoroughly washed with water. The passivation process begins. In the first case, the steel is immersed in an acid bath. In the second, gels, pastes, sprays and other ready-made chemical compositions are applied to the surface of the product. In the case of ready-made products, one more stage is provided - treatment with a passivator. This allows for the forced formation of an oxide film on stainless steel. The last stage consists of thoroughly washing the product.
The composition of stainless steel and the grade play an important role in the appearance of the product after chemical passivation. Some species are dark in color, while others are lighter. But regardless of this, this method of steel processing has a whole list of advantages:
improves resistance to corrosion; the surface of the product is uniformly smoothed; burrs, scratches, dents are removed; The service life of the products is significantly increased.
How to restore the corrosion protection of stainless steel
Passivation is the chemical treatment of a stainless steel product to restore its anti-corrosion resistance (in this case, the surface of the product is transferred to a passive state). After this treatment, the top layer of stainless steel oxidizes and forms a protective film that prevents corrosion of the material.
If we are talking about an ordinary household saucepan that suddenly begins to rust, then you can remove the stains using one of the commercially available special products containing a weak solution of nitric acid. But in order to prevent corrosion of the weld, it must be cleaned of scale. At home, this is done using a metal brush; at enterprises, mechanical cleaning is carried out using a hydro-sandblasting unit.
A widely used method for removing scale is by etching the metal with sulfuric acid or other substances. In order for the metal surface to be as smooth as possible after etching, it is necessary to ensure uniform action of the solution over the entire surface of the material.
Chemical treatment of stainless steel is carried out with various solutions, the composition of which depends on the type of steel. Thus, to passivate stainless steel with a high chromium content, nitric acid is used; for steel containing nickel, a mixture of nitric acid and sodium bichromate is used.
Products made from passivated steel can resist corrosion for a long time.
Passivation of stainless steel pipelines
The use of high-quality compressed air, high-purity technical gases (hydrogen, oxygen, nitrogen, helium), prepared water, or steam requires high-quality preparation of pipelines.
One of the most important stages in the installation of stainless steel pipelines is passivation. Passivation of pipelines allows for additional surface protection. Passivation of stainless pipelines is used in pharmaceutical production, microelectronics production, chemical plants, scientific organizations, and laboratories. In all industries where high quality of the transported medium is required (compressed air, special purity gases, treated water, etc.), it is recommended to passivate pipelines.
- Basis of the pipeline passivation process
- Why is passivation necessary?
- Features of passivation
When to passivate
For new equipment, a lot depends on its origin. High quality equipment is often immersed in nitric acid as one of the last steps in production and may only require a good cleaning to remove oil residues before first use. Manufacturers of less expensive equipment may skip the final immersion after running-in, welding, and polishing in order to save money.
Even if you know the source of your new equipment, I would lean toward both the thorough cleaning and passivation steps. Thorough cleaning is required to remove oils, polishes and other contaminants that could ruin your beer. But the extra step of passivation after cleaning is not a big price to pay compared to fairly expensive stainless steel equipment that can last you a lifetime.
You should also consider passivating your stainless steel at any time if you think you have damaged the protective chrome layer. This includes stubborn stains that require excessive cleaning, any scratches, dents on stainless steel, exposure to regular steel, steel or iron pads, or exposure to bleach cleaners. Also, if you use it for brewing often, it might not be a bad idea to passivate it every year or two just as a preventative measure.
Lastly, if you get rust or corrosion, it's important to fix it immediately. A mild abrasive such as Bar Keeper's Friend will help you remove rust and will also passivate the area to prevent further damage.
The essence and description of the metal passivation process
When passivating, the surfaces of metal products are treated with solutions of chemical compounds that have oxidizing properties. This role is most often played by acids, nitrites and solutions of chromium salts (less commonly, molybdenum). The solution is applied to the surface of metal workpieces by immersion or manually using special equipment. Solutions used for passivation usually consist of a main reagent and several additives that accelerate and stabilize the passivation process.
In general, the passivation process consists of the following stages:
- Mechanical cleaning of product surfaces.
- Chemical degreasing in a solution of sodium hydroxide and soda ash.
- Rinse in running hot and then cold water.
- Passivation for a specified time.
- Neutralization in soda ash solution.
- Rinsing by repeated immersion in running cold water.
- Dry in a drying cabinet or blowing warm air.
- Surface quality control after passivation is carried out visually or instrumentally. If the result is unsatisfactory, the passivation process is repeated, starting from step 1.
The given example describes the technological process of passivation using stationary production equipment. To passivate the surfaces of products at the site of their installation, hand-powered tools and devices are used (see photo below).
Stainless steel and rust
Steel is made from an alloy of iron and carbon, and carbon makes up only half or a little over a percent of its composition. In comparison, stainless steel is made from iron and chromium. Chromium contains approximately 10-30% of steel, and it is an important element that makes stainless steel resistant to corrosion.
The chromium in stainless steel reacts very quickly with oxygen, and actually forms a protective layer of chromium oxide on the surface of the steel. This chromium oxide prevents the formation of rust and corrosion. However, if the chromium layer is compromised for any reason, the iron in the steel can actually begin to corrode and rust.
Your stainless brewing equipment is generally very resistant to corrosion. However, if you expose it to bleach or other bleach cleaners, scratch it, over-clean it, or expose it to regular rusting steel pads or leave it in contact with regular steel, it can damage the protective layer. Bleaching agents can completely remove the protective layer. Excessive cleaning, especially with steel wool, can also undermine your oxidation layer. It is important to store regular steel in the same place as regular buckets, tools and some equipment other than your stainless steel equipment. Iron from ordinary steel tends to damage stainless steel (a property of iron) and destroy the oxidation layer. Do not place regular steel buckets or mixed metal tools or equipment in your stainless steel kettle after boiling.