Non-ferrous metallurgy is engaged in the extraction of non-ferrous metal ores, as well as the enrichment and smelting of pure metals and their alloys. Non-ferrous metals have many valuable properties: low density (magnesium, aluminum), high thermal conductivity (copper), corrosion resistance (titanium), etc. Conventionally, they are divided into heavy, light, noble and rare.
Metal groups
Heavy metals include substances that are highly dense. These are cobalt, chromium, copper, lead, etc. Some of them (lead, zinc, copper) are used in pure honey, but are usually used as alloying elements.
The density of light metals is less than 5 g/cm3. This group includes aluminum, sodium, potassium, lithium, etc. They are used as deoxidizers in the production of pure metals and alloys, and are also used in pyrotechnics, medicine, photographic equipment and other fields.
Noble metals are highly resistant to corrosion. This group includes platinum, gold, silver, osmium, palladium, rhodium, iridium and ruthenium. They are used in medicine, electrical engineering, instrument making, and jewelry.
Rare metals are combined into a separate group because they have special properties that are not characteristic of other metals. These are uranium, tungsten, selenium, molybdenum, etc.
There is also a group of widely used metals. It includes titanium, aluminum, copper, tin, magnesium and lead.
Alloys based on non-ferrous metals are cast and wrought. They differ in the technology for creating workpieces: parts are made from foundries by casting into metal or sand molds, and from deformed parts they are made into sheets, shaped profiles, wire and other elements. In this case, pressing, forging and stamping methods are used. Casting alloys belong to the metallurgy of heavy metals, wrought alloys belong to the metallurgy of light metals.
Non-ferrous metals and alloys: key characteristics and areas of application, marking
Non-ferrous metals are all existing metals with the exception of iron and its alloys (cast iron and steel - these are considered ferrous). Alloys of non-ferrous metals are mainly used as structural materials for various works. To understand their purpose, you should be able to correctly decipher the markings of alloys.
There is no unified system for marking non-ferrous metals and their alloys. However, they are always marked with letters and numbers, where the letters indicate whether the material belongs to one group or another, and numbers in different groups of materials or alloys can mean different things, for example:
- if it is a pure metal, then the degree of its purity;
- number of alloying elements;
- alloy number, etc.
Marking of copper and alloys based on it
When it comes to technical copper, the marking contains the letter M. Next are numbers indicating the degree of its purity. For example, M3 copper includes more impurities compared to M000 material. The letters at the end mean the following:
- B-oxygen-free material;
- R - deoxidized;
- K-cathode.
Copper in its pure form is often used as a conductor material for electrical purposes. The material lends itself well to soldering, deformation and welding, the only drawback is that it is difficult to cut.
In copper alloys, markings have an alphanumeric system by which their chemical composition can be determined. Thus, alloying elements are indicated by their initial letters, for example:
- K-silicon;
- Phosphorus;
- B-beryllium;
- O-tin, etc.
Titanium and titanium alloys
Titanium and its alloys are marked in accordance with existing GOST letters and numbers. There are no regularities in labeling. However, the key feature in this case is the mandatory presence of the letter “T”. The numbers indicate the conditional number of the titanium alloy.
Technical titanium can be marked as VT1−0 or VT1−00. Everything else means titanium alloys and has other markings, which are designated differently, and it will not be possible to list them all.
The key advantage of titanium and materials based on it is an excellent combination of properties such as:
- relatively low density;
- very high corrosion resistance;
- high mechanical strength.
But they also have disadvantages - they are scarce and expensive. For this reason, the use of this material in the refrigeration and food industries is limited. Titanium alloys are advantageously used in the following industries:
- shipbuilding;
- rocket science;
- aviation construction;
- chemical engineering;
- transport engineering.
The materials can be used at high temperatures up to 500 degrees. Products based on titanium materials are produced by pressure processing and also by casting. In composition, casting alloys correspond to wrought alloys, but when marked at the end they are indicated by the letter “L”.
Technical magnesium does not have the best properties, so it is not used as a construction material. But magnesium alloys, in accordance with standards, are divided into casting and wrought.
In accordance with GOST, foundries are marked as “ML”, as well as with a number indicating their conditional number. In some models, after the numbers there are the following lowercase letter designations:
- “pch” - increased purity;
- “he” is a general purpose material.
And deformable magnesium alloys are marked with the letters “MA”, as well as a number corresponding to the conditional number of the material. After the number there may also be the designation “pch”.
Magnesium materials have an excellent combination of properties such as:
- low density;
- high corrosion resistance;
- relatively high strength;
- good technological qualities.
Magnesium alloys are used to produce parts of simple and complex shapes that are highly resistant to corrosion. For example:
- fittings;
- neck;
- pump housings;
- gasoline tanks;
- brake wheel drums;
- steering wheels;
- farms, etc.
Aluminum and its alloys
Aluminum is a non-ferrous metal that has a silvery-white tint and melts at a temperature of 650°C. In the periodic table it corresponds to the symbol Al. This element ranks third in abundance among all rocks in the earth's crust (oxygen is in first place, silicon is in second place). Under atmospheric conditions, an oxide film forms on the surface of aluminum, preventing the occurrence of corrosion.
Important properties of aluminum:
- Low density - only 2.7 g/cm3 (for example, copper - 8.94 g/cm3).
- High electrical conductivity (37*106 S/m) and thermal conductivity (203.5 W/(m K)).
- Low strength in its pure form - 50 MPa.
- The structure of the crystal lattice is face-centered cubic.
The metal is easily processed by pressure. It is widely used in the electrical industry: electrical conductors are made from aluminum. In steel production it is used for deoxidation. Utensils are also made from aluminum, but they are not suitable for making pickles and storing fermented milk products - the element is unstable in alkaline and acidic environments. Some steel parts are coated with aluminum (aluminizing process) to improve their heat resistance. Due to its low strength, aluminum is practically not used in its pure form.
When marking aluminum, the letter A is used in combination with a number that indicates the metal content. For example, the A99 grade contains 99.95% aluminum, and the A99 grade contains 99.99%. There is also a special purity grade - A999, which contains 99.999% aluminum.
Wrought aluminum alloys
Wrought aluminum alloys are divided into hardenable and non-hardenable.
Strengthenable wrought aluminum alloys are duralumins (A-Cu-Mg system) and high-strength alloys (Al-Cu-Mg-Zn). High mechanical properties and low specific gravity allow these alloys to be widely used in mechanical engineering, especially in the manufacture of aircraft parts.
The main alloying elements for duralumin are magnesium and copper. These alloys are marked with the letter D with a number. D1 is used to make propeller blades, D16 is used for spars, frames, and aircraft skins, and D17 is used for fastening rivets.
High-strength alloys, in addition to aluminum, copper and magnesium, contain zinc. Designated by the letter B and a number, they are used for the manufacture of parts of complex configurations, helicopter blades, and highly loaded structures.
Non-hardening wrought aluminum alloys are alloys of aluminum with manganese (marked AMts1) and magnesium (AMg2 and AMg3). They are well processed by welding, drawing, rolling, hot and cold stamping. They are characterized by high ductility, but are not very durable. They are produced mainly in the form of sheets, which are used for the manufacture of products of complex shapes (rivets, frames, etc.).
Aluminum-based casting alloys
The most widely used casting alloys of aluminum and silicon, called silumins. They contain more than 4.5% silicon and are designated by the letters AK with a brand number. Silumins combine low specific gravity with high mechanical and casting properties. They are used for complex casting of auto, motorcycle and aircraft parts, as well as for the production of certain types of household appliances - meat grinders, heat exchangers, sanitary fittings, etc.
Copper alloys
Copper is a non-ferrous metal that has a red tint on the surface and pink when broken. In the periodic table D.I. Mendeleev is designated by the symbol Cu. In its pure form, the metal has a high degree of ductility, electrical and thermal conductivity, and is also characterized by resistance to corrosion. This allows the use of copper and its alloys for roofing of critical buildings.
Important properties of the metal:
- Melting point - 1083°C.
- The structure of the crystal lattice is face-centered cubic.
- Density - 8.94 g/cm3.
Due to its ductility, copper can be easily processed by pressure, but is difficult to cut. Due to high shrinkage, the metal has low casting properties. Any impurities, with the exception of silver, have a great effect on the substance and reduce its electrical conductivity.
When marking copper, the letter M is used with a number that indicates the grade. The lower the brand number, the more pure substance it contains. For example, M00 contains 99.99% copper, and M4 - 99%.
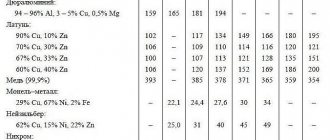
The most widely used in technology are two groups of copper alloys - bronze and brass.
Bronze
Bronzes are copper-based alloys in which the alloying element is any metal except zinc. The most commonly used alloys are copper with lead, tin, aluminum, silicon and antimony.
All bronzes according to their chemical composition are divided into tin and special, or tin-free, that is, not containing tin.
Tin bronzes are distinguished by the highest casting, mechanical and antifriction properties, and also have increased resistance to corrosion. Due to the high cost of tin, these alloys are used to a limited extent.
Special bronzes are often used as substitutes for tin, and some have better processing properties. The following types of special bronzes are distinguished:
- Aluminum. They contain from 5% to 11% aluminum, as well as manganese, nickel, iron and other metals. These alloys have higher mechanical properties than tin bronzes, but their casting properties are lower. Aluminum bronzes are used for the manufacture of small critical parts.
- Leady. They contain about 30% lead. These alloys have high antifriction properties and are therefore widely used in the production of bearings.
- Siliceous. These bronzes contain approximately 4% silicon and are alloyed with nickel and manganese. In terms of their mechanical properties, they almost correspond to steel. They are mainly used for the manufacture of spring elements in shipbuilding and aviation.
- Beryllium. They contain up to 2.3% beryllium and are characterized by high elasticity, hardness and wear resistance. These bronzes are used for springs that operate in aggressive environments.
All bronzes have good anti-friction properties, corrosion resistance, and high casting properties, which make it possible to use alloys for making monuments, casting bells, etc.
When marking bronzes, the initial letters Br are used, followed by the first letters of the names of the base metals, indicating their percentage content. For example, the BrOF8-0.3 alloy includes 8% tin and 0.3% phosphorus.
Brass
Brasses are alloys of copper and zinc with the addition of other metals - aluminum, lead, nickel, manganese, silicon, etc. Simple brasses contain only copper and zinc, and multicomponent alloys include from 1% to 8% of various alloying elements that are added to improve various properties.
- Manganese, nickel and aluminum increase the alloy's corrosion resistance and mechanical properties.
- Thanks to the addition of silicon, the alloy becomes more fluid in the liquid state and easier to weld.
- Lead makes cutting easier.
The percentage of zinc in any brass does not exceed 50%. These alloys are less expensive than pure copper, and due to the addition of zinc and alloying elements, they have greater corrosion resistance, strength and toughness, and are also characterized by high casting properties. Brass is used for the manufacture of parts by rolling, drawing, stamping, etc.
When marking plain brass, the letter L and a number indicating the copper content are used. For example, grade L96 contains 96% copper. For multi-component brasses, a complex formula is used: the letter L, then the first letters of the base metals, a number indicating the copper content, and then the composition of other elements in order. For example, brass LAMsh77-2–0.05 contains 77% copper, 2% aluminum, 0.05% arsenic, and the rest is zinc.
Production of certain types
Copper production
This non-ferrous metal is obtained from copper ores. Its content in these compounds ranges from 1 to 6%. With a copper composition of less than 1%, its extraction at the current level of technology development does not seem to be profitable.
Copper is obtained in two ways:
- hydrometallurgical;
- pyrometallurgical.
The first method is less common, since when using it it is not possible to extract other elements from the ore.
The pyrometallurgical copper mining method consists of several successive stages:
- Preparing ore for smelting through beneficiation and further roasting. This allows you to obtain a copper concentrate.
- Subsequent roasting is required to reduce the amount of sulfur.
- Melting for matte. By smelting copper concentrates it is possible to obtain matte or sulfides of copper and iron.
Matte conversion is also carried out. This stage consists of blowing air inside a special copper smelting converter onto the resulting matte, which allows the iron to be separated into slag and obtain blister copper.
And finally - refining. Blister copper is subjected to fire melting and electrolytic refining, which ultimately results in a product whose purity is 99.97–99.99%.
Aluminum production
Aluminum is produced by the electrolysis of alumina. The process includes several stages.
Obtaining pure alumina or aluminum oxide. This process involves treating bauxite (ores containing metal) with alkaline solutions. The result is the precipitation of aluminum hydroxide.
Preparation of cryolite - its production consists of processing fluorspar to obtain hydrofluoric acid and further release of fluoroaluminum acid. By means of soda, cryolite is released in the form of sediment.
Electrolysis of alumina - the result of this process is the production of raw aluminum.
Refining – by blowing molten raw material with chlorine, pure aluminum is extracted.
Magnesium production
Magnesium is extracted through an electrolysis reaction. The raw materials are molten metal salts (carnallite, magnesite, dolomite, bischofite). The electrolyte is based on magnesium chloride. Additionally, sodium chloride, calcium and potassium are used.
After the reaction, a rough metal containing up to 5% impurities settles on the anode. Their removal occurs through a refining process using fluxes. All non-metallic components are converted into slag, and pure metal is poured into molds.
Titanium production
Titanium and its alloys are superior in quality to alloy steels. The titanium production process is hampered by its increased activity, especially as temperatures rise.
Its peculiarity is the ability to react with many metals, which requires compliance with certain conditions to obtain pure titanium.
The method used to obtain titanium is called magnetothermy. It consists of the following operations.
Isolation of titanium concentrate by beneficiation of ore containing such metal.
Slag production - at this stage, the separation of iron oxides from titanium oxides occurs.
Obtaining titanium tetrachloride - to obtain titanium metal, the use of titanium chloride is required, obtained by chlorinating slag.
Reduction through magnesium - the reduction process takes place at very high temperatures - close to 1 thousand degrees. A reactor where magnesium is melted and titanium vapor is supplied. During metallization, it settles on the walls, and molten magnesium is removed through a tap hole.
Separation of the mass in a vacuum - the titanium obtained as a result of the previous step in the form of a sponge mass must be heated using a vacuum, which will allow the separation of pure metal.
Features of raw materials
All non-ferrous metals have a number of characteristics that must be taken into account when processing or using them.
A number of elements have increased thermal conductivity and specific heat capacity:
- copper;
- magnesium;
- aluminum.
When welding, the joint quickly cools, which will require the use of powerful sources, especially heat during welding.
Some elements change their mechanical properties when suddenly heated. Their decline is observed. At the same time, the metal itself becomes easily destroyed by impacts or other mechanical impacts.
All non-ferrous metals easily interact with gases, except inert ones. This feature is typical for refractory non-ferrous metals.
Magnesium and its alloys
Magnesium is a non-ferrous metal that has a silvery tint and is represented by the symbol Mg in the periodic table.
Important properties of magnesium:
- Melting point - 650°C.
- Density - 1.74 g/cm3.
- Hardness - 30-40 HB.
- Relative elongation - 6-17%.
- Tensile strength - 100-190 MPa.
The metal has high chemical activity and is not resistant to corrosion in atmospheric conditions. It cuts well, absorbs shock loads and dampens vibrations. Since magnesium has low mechanical properties, it is practically not used for structural purposes, but is used in pyrotechnics, the chemical industry and metallurgy. It often acts as a reducing agent, alloying element and deoxidizing agent in the manufacture of alloys.
When marking, the letters Mg are used with numbers that indicate the percentage of magnesium. For example, the Mg96 brand contains 99.96% magnesium, and Mg90 contains 99.9%.
Magnesium-based alloys are characterized by high specific strength (tensile strength - up to 400 MPa). They are well cut, ground, polished, forged, pressed, rolled. The disadvantages of magnesium alloys are low corrosion resistance, poor casting properties, and a tendency to ignite during manufacturing.
Wrought magnesium alloys
The most common are three groups of magnesium-based alloys.
Magnesium alloys alloyed with manganese
They contain up to 2.5% manganese and are not hardened by heat treatment. They have good corrosion resistance. Since these alloys are easy to weld, they are used for welded parts of simple configurations, as well as for fittings, oil and gasoline systems that do not experience heavy loads. Among this group are the MA1 and MA8 alloys.
Alloys of the Mg-Al-Zn-Mn system
The composition of these alloys, in addition to magnesium and manganese, includes aluminum and zinc. They significantly increase strength and ductility, making the alloys suitable for the manufacture of stamped and forged parts of complex shapes. This group includes brands MA2-1 and MA5.
Alloys of the Mg-Zn system
Alloys based on magnesium and zinc are additionally alloyed with cadmium, zirconium and rare earth metals. These are high-strength magnesium alloys that are used for parts subject to high loads (in airplanes, cars, machine tools, etc.). This group includes alloys of the MA14, MA15, MA19 grades.
Casting magnesium alloys
The most common group of cast magnesium alloys belongs to the Mg-Al-Zn system. These alloys practically do not absorb thermal neutrons, therefore they are widely used in nuclear technology. They are also used to make parts for airplanes, rockets, and cars (cabin doors, instrument housings, fuel tanks, etc.). Alloys of magnesium, zinc and aluminum are used in instrument making and in the manufacture of housings for electronic equipment. This group includes brands ML5 and ML6.
High-strength cast magnesium alloys have better mechanical and technological properties. They are used in aviation for the manufacture of loaded parts. This group includes alloys ML12 (magnesium, zinc and zirconium), ML8 (magnesium, zinc, zirconium and cadmium), ML9 (magnesium, zirconium, neodymium), ML10 (magnesium, zinc, zirconium, neodymium).
Non-ferrous metals and their applications
Non-ferrous metals and their alloys are in great demand and are widely used in all sectors of industry and agriculture. These include all metals except iron and its derivatives, which are classified as ferrous metals.
Almost all non-ferrous metals have the following properties:
- Resistant to corrosion and significant temperature changes;
- Plasticity;
- Versatility of application.
In addition, an important feature of non-ferrous metals is that their properties can be changed by hardening, artificial aging or heat treatment. They are also well processed by stamping, rolling, forging, welding, soldering, pressing and cutting.
The most valuable non-ferrous metals are: Aluminum; Copper; Nickel; Tin; Lead; Zinc; Magnesium.
Aluminum
Possessing high electrical conductivity, aluminum in its pure form is widely used where this property is important, for example, for the manufacture of power line wires.
Aluminum alloys are also widely used, which are divided into two groups: hardenable and non-hardenable.
Hardenable alloys that undergo heat treatment are known under the names duralumin and avial; they contain copper, zinc and a certain combination of magnesium and silicon.
In addition to heat treatment, such alloys are subjected to natural aging and hardening, which increases their strength characteristics. These types of alloys are used to create high-strength, low-weight structures for use in the aerospace industry.
Alloys that cannot be strengthened by heat treatment are widely used in transport engineering for the manufacture of components for a wide variety of vehicles.
Copper
Copper became the first metal that man began to use, and this most likely happened many millennia BC. In addition, copper was the first material that was used to transmit electricity. Its main technical characteristics are high electrical conductivity and malleability.
Pure copper is widely used in the electrical industry for the manufacture of cable products and various types of wires. It is also used in the production of electric generators, radio equipment, telegraph and telephone equipment.
In other industries, its alloys are more often used. Particularly popular are brasses that contain zinc and other elements to impart the necessary properties.
They have excellent mechanical characteristics and are easy to process, therefore they are widely used in the chemical industry and mechanical engineering for the manufacture of various containers and pipelines.
They are also used everywhere for the production of household goods for various purposes.
In addition to them, bronzes containing tin as the main component of the alloy are widely used.
Nickel
Pure nickel is used as a protective anti-corrosion coating for surfaces against the effects of chemically active substances.
In addition, various boilers, tanks and crucibles are made from it, which have high corrosion resistance and are used in the chemical, textile, and food industries. Nickel is widely used in the production of various types of batteries and electrodes for fuel cells.
Some applications use powdered nickel as a catalyst for chemical processes. For example, it is used in the hydrogenation reactions of alcohols, cyclic aldehydes, aromatic and unsaturated hydrocarbons.
Tin
Pure tin is mainly used to produce tinplate, which is used to make tin cans.
Alloys made from this non-ferrous material are very popular in various industries. For example, when printing books, fonts are used that are cast from garth, which is an alloy of tin with lead and antimony.
Babbitt, produced by alloying tin with lead, antimony and copper, is very popular. A huge number of parts are made from this alloy, in particular bearings, the working surface of which is highly stable and has a low coefficient of friction.
Lead and zinc
Although lead and zinc are mined from the same natural deposits, their applications differ significantly. Lead's resistance to aggressive influences allows it to be used as protective coatings for telephone and telegraph wires. In chemical production, special equipment is made from it.
Pure zinc is often used to make galvanized iron. Both metals are widely used in various alloys for the manufacture of equipment components in mechanical engineering, metallurgy, medicine and other sectors of the national economy.
Zinc and its alloys
Zinc is a non-ferrous metal of a gray-bluish hue. In D.I. Mendeleev’s system it is designated by the symbol Zn. It has high viscosity, ductility and corrosion resistance. Important properties of the metal:
- Low melting point - 419 ° C.
- High density - 7.1 g/cm3.
- Low strength - 150 MPa.
In its pure form, zinc is used to galvanize steel to protect against corrosion. Used in printing, printing and electroplating. It is often added to alloys, mainly copper.
There are the following grades of zinc: TsV00, TsV0, TsV, Ts0A, Ts0, Ts1, Ts2 and Ts3. TsV00 is the purest grade with a zinc content of 99.997%. The lowest percentage of pure substance in the Ts3 brand is 97.5%.
Wrought zinc alloys
Wrought zinc alloys are used to produce parts using drawing, pressing and rolling methods. They are processed hot at temperatures from 200 to 300? C. The alloying elements are copper (up to 5%), aluminum (up to 15%) and magnesium (up to 0.05%).
Wrought zinc alloys are characterized by high mechanical properties, due to which they are often used as substitutes for brass. They have high strength with good ductility. Zinc, aluminum and copper alloys are the most common as they have the highest mechanical properties.
Cast zinc alloys
In cast zinc alloys, alloying elements also include copper, aluminum and magnesium. Alloys are divided into 4 groups:
- For injection molding.
- Anti-friction.
- For centrifugal casting.
- For chill casting.
The ingots are easily polished and accept electroplating. Cast zinc alloys have high fluidity in the liquid state and form dense castings when solidified.
Casting alloys are widely used in the automotive industry: they are used to make housings for pumps, carburetors, speedometers, and radiator grilles. Alloys are also used for the production of certain types of household appliances, fittings, and instrument parts.
In Russia, non-ferrous metallurgy is one of the most competitive industries. Many domestic companies are world leaders in the nickel, titanium, and aluminum sub-sectors. These achievements were made possible thanks to large investments in non-ferrous metallurgy and the use of innovative technologies.