Tungsten stands out among metals not only in its refractoriness, but also in its mass. The density of tungsten under normal conditions is 19.25 g/cm³, which is approximately 6 times greater than that of aluminum. Compared to copper, tungsten is 2 times heavier. At first glance, high density may seem like a disadvantage, because products made from it will be heavy. But even this feature of the metal has found its application in technology. Useful properties of tungsten due to its high density:
- The ability to concentrate a large mass in a small volume.
- Protection against ionizing radiation (radiation).
The first property is explained by the internal structure of the metal. The nucleus of an atom contains 74 protons and 110 neutrons, i.e. 184 particles. In the Periodic Table of Chemical Elements, in which atoms are arranged in order of increasing atomic mass, tungsten is in 74th place. For this reason, a substance consisting of heavy atoms will have a large mass. The ability to protect against radiation is inherent in all materials with high density. This is due to the fact that ionizing radiation, when encountering any obstacle, transfers part of its energy to it. Denser substances have a higher concentration of particles per unit volume, so ionizing rays undergo more collisions and therefore lose more energy. The use of metal is based on the above properties.
What is the maximum valency that tungsten can exhibit?
Table. Oxidation states of chemical elements. ... Valence
chemical elements.
| Ordinal number of a chemical element, also known as atomic number, also known as: charge number of the atomic nucleus, also known as: atomic number | 74 |
| Russian/English name | Tungsten /Tungsten |
| Chemical symbol | W |
| Valence | (+2), (+3), (+4), (+5), +6 |
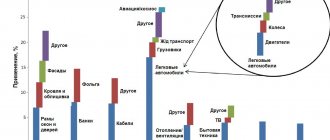
Processed products
Due to its unique properties, primarily hardness and refractoriness, tungsten has found a wide range of applications since its discovery. As a refractory material, it is widely used in metallurgy. Although other industries cannot do without such valuable material.
Lighting
Due to their low electrical conductivity and low evaporation rate, at one time tungsten filaments made it possible to make a technical revolution in the entire industry of creating electric lighting devices, and also began to be used in the manufacture of electronic vacuum devices.
Shells
The high level of density of this material, reaching up to 19.3 g/cm3, along with its strength, provided gunsmiths with an excellent means of destroying armor. Today, tungsten is one of the main chemical elements that make up the heavy alloy cores of armor-piercing bullets and shells.
Tungsten scrap
Electrodes
Non-consumable tungsten electrodes are used as a welding material for a process carried out using gases. Helium or argon protects the connection point from atmospheric influences, and the electrode at this time withstands significant temperatures and a long service life. This allows you to create optimal working conditions while avoiding unnecessary costs.
Main sources of scrap
The paradox of this rare metal is that it can be found even in an ordinary residential building.
It is found in incandescent light bulbs and in the windings of electric furnaces. These are the main sources of tungsten for ordinary citizens and individuals.
However, it is very, very difficult to accumulate a sufficient amount to sell this non-ferrous metal from these sources.
Therefore, those who want to make a profit from several dozen light bulbs usually turn to resellers.
Recently, incandescent lamps containing tungsten have become unpopular. This is because energy-saving light bulbs or LED light bulbs that do not contain this metal have come into use.
Tungsten is also contained in pobedite tips of concrete drills, which are also found in everyday life. It contains more of the main element, but it is not pure metal, but one of its alloys - “pobedit” or VK with cobalt.
Recyclable materials are sold by state and private enterprises that specialize in metal processing. They accept used tools made from high-speed steels:
- incisors;
- cutters;
- borax;
- cutting discs and so on.
Do not forget that tungsten is common in the form of a finished product:
And also in the form of production waste:
Tungsten dust is weighed in grams and, accordingly, the price is also set per gram.
Nomenclature of metal grades
Based on tungsten or with its participation, metallurgists smelt products of dozens of names and brands.
Among the most common are pure tungsten (HF) and alloy with rhenium (HR).
The classification of tungsten grades is based on the composition of the additives:
| Brand name | Type of additive |
| VA | Aluminum + siliceous alkali |
| VM | Thorium + siliceous alkali |
| IN AND | Yttrium oxide |
| VT | Thorium oxide |
| VL | Lanthanum oxide |
Tungsten filament manufacturing technology and its history.
The production volumes of tungsten wire have a small share among all tungsten applications, but the development of the technology for its production played a key role in the development of powder metallurgy of refractory compounds.
Since 1878, when Swan demonstrated the eight- and sixteen-candle carbon lamps he had invented in Newcastle, a search had been going on for a more suitable material for making incandescent filaments. The first coal lamp had an efficiency of only 1 lumen/watt, which was increased over the next 20 years by modifications in coal processing methods by two and a half times. By 1898, the light output of such bulbs was 3 lumens/watt. In those days, carbon filaments were heated by passing an electric current in an atmosphere of heavy hydrocarbon vapors. During the pyrolysis of the latter, the resulting carbon filled the pores and irregularities of the thread, giving it a bright metallic shine.
At the end of the 19th century. von Welsbach was the first to produce metal filament for incandescent lamps. He made it from osmium (Tm = 2700° C). Osmium filaments had an efficiency of 6 lumens/watt, however, osmium is a rare and extremely expensive platinum group element, so it was not widely used in the manufacture of household devices. Tantalum, with a melting point of 2996° C, was widely used in the form of drawn wire from 1903 to 1911 thanks to the work of von Bolton of Siemens and Halske. The efficiency of tantalum lamps was 7 lumens/watt.
Tungsten began to be used in incandescent lamps in 1904 and replaced all other metals in this capacity by 1911. A conventional incandescent lamp with a tungsten filament has a glow of 12 lumens/watt, and lamps operating under high voltage - 22 lumens/watt. Modern tungsten cathode fluorescent lamps have an efficiency of about 50 lumens/watt.
In 1904, they tried to apply the wire drawing process developed for tantalum to more refractory metals such as tungsten and thorium. The rigidity and lack of malleability of tungsten did not allow the process to proceed smoothly. However, it was later shown in 1913–1914 that molten tungsten could be rolled out and drawn using a partial reduction procedure. An electric arc was passed between a tungsten rod and a partially molten tungsten droplet placed in a graphite crucible coated inside with tungsten powder and located in a hydrogen atmosphere. Thus, small drops of molten tungsten were obtained, about 10 mm in diameter and 20–30 mm in length. Although with difficulty, it was already possible to work with them.
During the same years, Just and Hannaman patented a process for making tungsten filaments. Fine metal powder was mixed with an organic binder, the resulting paste was passed through dies and heated in a special atmosphere to remove the binder, resulting in a thin thread of pure tungsten.
In 1906–1907 the well-known extrusion process was developed and used until the early 1910s. Very finely ground black tungsten powder was mixed with dextrin or starch until a plastic mass was formed. Using hydraulic pressure, this mass was forced through thin diamond sieves. The resulting thread was strong enough to be wound onto spools and dried. Next, the threads were cut into “pins,” which were heated in an inert gas atmosphere to a red-hot temperature to remove residual moisture and light hydrocarbons. Each “pin” was secured in a clamp and heated in a hydrogen atmosphere until it glowed brightly by passing an electric current. This led to the final removal of unwanted impurities. At high temperatures, individual small particles of tungsten fuse and form a homogeneous solid metal filament. These threads are elastic, although fragile.
At the beginning of the 20th century. Yust and Hannaman developed another process that was notable for its originality. A carbon filament with a diameter of 0.02 mm was coated with tungsten by heating in an atmosphere of hydrogen and tungsten hexachloride vapor. The thread coated in this way was heated to a bright glow in hydrogen at reduced pressure. In this case, the tungsten shell and the carbon core were completely fused with each other, forming tungsten carbide. The resulting thread was white and brittle. The filament was then heated in a stream of hydrogen, which reacted with the carbon, leaving a compact filament of pure tungsten. The threads had the same characteristics as those obtained during the extrusion process.
In 1909, the American Coolidge managed to obtain malleable tungsten without the use of fillers, but only with the help of reasonable temperature and mechanical processing. The main problem in producing tungsten wire was the rapid oxidation of tungsten at high temperatures and the presence of a grain structure in the resulting tungsten, which led to its brittleness.
Modern production of tungsten wire is a complex and precise technological process. The starting material is powdered tungsten obtained by reducing ammonium paratungstate.
Tungsten powder used for wire production must be of high purity. Typically, tungsten powders of different origins are mixed to homogenize the quality of the metal. They are mixed in mills and, to avoid oxidation of the metal heated by friction, a stream of nitrogen is passed into the chamber. The powder is then pressed in steel molds using hydraulic or pneumatic presses (5–25 kg/mm2). When contaminated powders are used, the compact becomes brittle and a fully oxidizable organic binder is added to eliminate this effect. At the next stage, preliminary sintering of the bars is carried out. When heating and cooling compacts in a hydrogen flow, their mechanical properties improve. The compacts still remain quite fragile, and their density is 60–70% of the density of tungsten, so the bars are subjected to high-temperature sintering. The rod is clamped between contacts cooled by water, and in an atmosphere of dry hydrogen, a current is passed through it to heat it almost to the melting point. Due to heating, tungsten is sintered and its density increases to 85–95% of the crystalline density, at the same time the grain sizes increase and tungsten crystals grow. This is followed by forging at high (1200–1500° C) temperatures. In a special apparatus, the rods are passed through a chamber, which is compressed with a hammer. During one pass, the diameter of the rod decreases by 12%. When forged, tungsten crystals elongate, creating a fibrillar structure. After forging, wire drawing follows. The rods are lubricated and passed through diamond or tungsten carbide screens. The degree of drawing depends on the purpose of the resulting products. The diameter of the resulting wire is about 13 microns.
Types of wire for stainless steel 12x18n10t
To weld stainless steel parts, it is necessary to use argon arc welding and an additive made of the same material. It may have different properties that may be suitable for other cases. Long products are produced from steel 12×18N10T. The filler material of this grade must meet the requirements of GOST 18143-72.
Welding filler wire has found its use in the mechanical engineering and food industries, construction, etc. It has not only high corrosion resistance, but also resistance to chemically aggressive environments. It contains a sufficient amount of chromium, which protects it from rust.
For welding work, products produced using cold drawing technology are used. It has a fairly low price and at the same time, this treatment preserves all its properties. This wire ensures the quality of the seam when processing any material.
Stainless steel wire 12Х18Н10Т
Thus, water supply systems are often assembled from pipeline fittings made from this grade of steel. When assembling and repairing, it is considered optimal to use a welding additive of the 12Х18Н10Т brand.
This steel grade is available in several versions. For its production, hot or cold rolling technologies are used. They make it possible to obtain a product with a diameter from 0.2 to 6 mm. When using wire of this brand, it is necessary to take into account that it can change some of its parameters based on the diameter.
Welding stainless steel parts is a complex technological process and if its rules are violated, the result can be a large number of substandard products. To avoid this, it is necessary to make the right choice of wire material. Wire made from steel 12Х18Н10Т is a specific product and quite possibly may not be suitable for most types of alloy steel. The main rule for choosing a material for welding is the identity of the chemical composition. The good thing about wire made from this wire is that the industry produces a wide range of products and, as a rule, there are no problems with selection. By the way, when welding, pre-heating and gradual cooling may be required. Heating is carried out using a gas burner.
Use based on ability to protect against radiation
Tungsten collimators in surgery.
- According to this criterion, tungsten alloys are ahead of cast iron, steel, lead and water, which is why collimators and protective screens that are used in radiotherapy are made from metal. Tungsten alloys are not subject to deformation and are highly reliable. The use of multi-leaf collimators makes it possible to direct radiation to a specific area of the affected tissue. During therapy, X-rays are first taken to localize the location and determine the nature of the tumor. Then the collimator blades are moved by an electric motor to the desired position. 120 petals can be used, with the help of which a field is created that follows the shape of the tumor. Next, high radiation rays are directed to the affected area. In this case, the tumor receives radiation by rotating a multileaf collimator around the patient. To protect neighboring healthy tissues and the environment from radiation, the collimator must be highly accurate.
- Special ring collimators made of tungsten have been developed for radiosurgery, the irradiation of which is directed to the head and neck. The device provides high-precision focusing of gamma radiation. Tungsten is also included in plates for computed tomographs, shielding elements for detectors and linear accelerators, dosimetric equipment and non-destructive testing instruments, and containers for radioactive substances. Tungsten is used in drilling devices. Screens are made from it to protect submersible instruments from X-ray and gamma radiation.
ORIGIN
Tungsten occurs in nature mainly in the form of oxidized complex compounds formed by tungsten trioxide WO3 with oxides of iron and manganese or calcium, and sometimes lead, copper, thorium and rare earth elements. Wolframite (iron and manganese tungstate nFeWO4 * mMnWO4 - ferberite and hübnerite, respectively) and scheelite (calcium tungstate CaWO4) are of industrial importance. Tungsten minerals are usually embedded in granite rocks, so the average tungsten concentration is 1-2%.
Kazakhstan, China, Canada and the USA have the largest reserves; deposits are also known in Bolivia, Portugal, Russia, Uzbekistan and South Korea. World tungsten production is 49-50 thousand tons per year, including 41 in China, 3.5 in Russia; Kazakhstan 0.7, Austria 0.5. Main exporters of tungsten: China, South Korea, Austria. Main importers: USA, Japan, Germany, UK. There are also tungsten deposits in Armenia and other countries.
Types of wire for semi-automatic machines
The selection of welding wire for semi-automatic machines should be carried out for a specific type of metal being joined. The use of filler consumables significantly improves the quality of the seam and prevents the formation of pores and irregularities in the joint.
The main advantages of using an additive when performing welding work are presented:
- acceleration of the welding process;
- ease of use in industrial applications;
- a significant reduction in the likelihood of defects due to the lack of additive coating;
- a large selection of consumables, allowing you to select the optimal additive for each specific case;
- low level of slag formation during welding.
Disadvantages of using a filler component in welding:
- the need for constant protection;
- difficulty in storing large skeins;
- difficulty in selecting the optimal diameter of the additive;
- the need to constantly use flux.
Wire grades table.
All types of wire for welding are usually divided into:
- Copper-plated. This type of wire is used for welding carbon and low-alloy steel parts. Copper-plated steel filler components provide high-quality welds and are characterized by a low metal spatter coefficient.
- Powder. Additive components of such grades are made in the form of a hollow tube made of low-carbon steel. Inside the container there are deoxidizers and slag-forming substances, which ensure comfortable use of semi-automatic welding without shielding gas. Flux cored filler wires help to significantly reduce slag formation and reduce weld processing time.
- Solid section. This type of wire differs from ordinary wire in that welding electrodes are made from it.
- Non-copper-plated. Additives of this type are used primarily for working with products made of low-carbon steel.
- Activated. Powder additives used during welding in a carbon dioxide atmosphere.
- Gas welding. To work with carbon and low-carbon grades of steel, it is best to use gas welding filler components.
- Aluminum. One of the few types of wires suitable for welding aluminum parts. When working with aluminum filler, low porosity of welds is observed. Such additives are actively used in the shipbuilding and dairy industries.
- Made from stainless steel. The filler component allows you to weld stainless steel products and prevent corrosion of the resulting weld.
- Flux. This type of filler wire is widely used for joining medium carbon, low carbon and carbon steels. Due to the presence of a built-in flux, such additives can be used when welding without shielding gas.
- Alloyed. One of the best components that allows welding work in any gas mixtures and with any types of metals.
To summarize: how to profitably sell tungsten
In order to get as much profit from the delivery of tungsten scrap, you need to prepare the goods for delivery .
The highest price can be obtained if:
- clean the raw materials (make sure that there are no other metals, earth or dirt in it);
- sort recyclables by type and material;
- compress the product using any suitable press;
- collect as much metal as possible , because in most companies the fundamental factor in increasing the price is the amount of scrap delivered;
- consider at least five large enterprises engaged in scrap collection and choose the most favorable price.
Thus, by donating tungsten, you not only receive significant benefits, but also help the industry, which is experiencing a shortage of this rare but necessary metal.
Areas of application of nichrome wire
The specific properties of nichrome have found their application in various fields of activity, both domestic and industrial.
All kinds of varieties of this alloy are used as heating elements in various devices: electric furnaces, dryers, thermocouples, as well as in ceramic products, acting as a supporting frame.
Nichrome wire, as the main executive element of electric heating devices, in most cases is a spiral through which an electric current of a given value is passed. This form is recognized as the most optimal for these devices, as it allows for greater heat transfer by increasing the length of the conductive element. The high degree of plasticity of the presented material significantly increases the service life of such devices, due to its high resistance to deformation.
Where can you get nichrome wire at home? This alloy is widely used in various household appliances. Examples of these include electric heaters, hair dryers, soldering irons, toasters and ovens. In addition, they are used in electronic cigarettes.
Nichrome wire: selection criteria
The implementation of a project to create various types of electrothermal equipment requires a thorough analysis of the nominal operating parameters, which will serve as the starting point in calculating the key indicators of nichrome wire.
First of all, it is necessary to calculate the electrical resistance of the working element. It depends on three physical quantities, namely: the resistivity of the material, its length and cross-sectional area. The formula for calculating active resistance is as follows: R = ρ l / S.
To obtain the missing data, it is necessary to calculate the length of the spiral. Depending on the voltage applied to it, you should choose the most optimal value for the length of the wire, its diameter, and the size of the core. In order to save yourself from time-consuming calculations, you can use a summary table that shows the values of the spiral length, depending on the diameter of the wire and core for nichrome with a diameter of 0.2 to 0.5 mm.
| D 0.2 mm | D 0.3 mm | D 0.4 mm | D 0.5 mm | ||||
| Core D, (mm) | Spiral length (cm) | Core D, (mm) | Spiral length (cm) | Core D, (mm) | Spiral length (cm) | Core D, (mm) | Spiral length (cm) |
| 1,5 | 49 | 1,5 | 59 | 1,5 | 77 | 2 | 64 |
| 2 | 30 | 2 | 43 | 2 | 68 | 3 | 46 |
| 3 | 21 | 3 | 30 | 3 | 40 | 4 | 36 |
| 4 | 1 | 4 | 22 | 4 | 28 | 5 | 30 |
| 5 | 13 | 5 | 18 | 5 | 24 | 6 | 26 |
| 6 | 20 |
The presented table is applicable for calculating the length of the spiral when using a voltage of 220 V. For example, for a wire with a diameter of 0.4 mm and a core diameter of 3 mm, the length of the spiral for a household electrical network will be 40 cm. It is worth noting that using this table it will not be difficult to calculate the desired value for a voltage of 380 V. To do this, it is enough to select the necessary data and make a proportion of the following form: 220 V - 40 cm / 380 V - x.
In the event that you do not have a measuring tool at hand and it is not possible to determine the diameter of the wire, you can always use a simple but at the same time effective method. To do this, just take an ordinary pencil and wind a wire around it, pressing tightly, turn by turn. In the event that 10 turns of the spiral fit into 1 mm. pencil length, then the wire diameter will be 1\10 mm.
World reserves
The world's confirmed reserves of tungsten amount to 2.6 million tons. Identified resources amount to 12.5 million tons. Inferred resources are estimated at 9.5 million tons. Over 60 countries around the world have deposits of this metal:
- China – 7.5 million tons.
- Kazakhstan – 3.1 million tons.
- Russia – 3 million tons.
- Canada – 1.7 million tons.
- USA – 0.8 million tons.
- Australia – 0.7 million tons.
- Bolivia – 0.5 million tons.
It should be noted that a number of countries in the world community have deposits that are unsuitable for development due to their unprofitability. While the five leading ones have more than 70% of developed reserves in their territories.
Peculiarities
For the manufacture of tungsten wire - GOST 18903-73 - forged rods are used. During drawing, a gradual decrease in temperature is carried out. The product is then cleaned by annealing and electrolytic polishing.
The raw material for the manufacture of this type of wire product is the most refractory metal. This material is heat-resistant and durable, it is not afraid of acidic and alkaline environments. Such characteristics make it possible to use tungsten wire to produce parts intended for use in heated conditions, as a result of which they do not lose their original properties.
The mechanical parameters characteristic of this type of wire product (increased hardness, resistance to wear during heating, low thermal expansion), exceeding many similar materials, make tungsten products very popular.
This type of rolled metal is distinguished by a high modulus of elasticity, excellent ohmic resistance, and good thermal conductivity. This is a durable and reliable material that can withstand extreme operating conditions, which makes it indispensable in various manufacturing industries.
There are several brands of such wire. The classification is carried out in accordance with the cross-sectional diameter and the percentage of tungsten in the material.
The wire diameter can range from 12.5 to 500 microns.
The most popular brand is BA. The BRN brand is used for the production of cathodes for electronic devices.
The VM and VT tungsten metal grades are also in demand.
It is the brand that determines the scope of application of the material.
Properties of a simple substance.
Tungsten metal has a light gray color. After carbon, it has the highest melting point of all simple substances. Its value is determined within the range of 3387–3422° C. Tungsten has excellent mechanical properties at high temperatures and the lowest coefficient of expansion among all metals. Boiling point 5400–5700° C. Tungsten is one of the heaviest metals with a density of 19250 kg/m3. The electrical conductivity of tungsten at 0° C is about 28% of the electrical conductivity of silver, which is the most electrically conductive metal. Pure tungsten is quite easy to process, but it usually contains impurities of carbon and oxygen, which gives the metal its well-known hardness.
Tungsten has a very high tensile and compressive modulus, very high thermal creep resistance, high thermal and electrical conductivity, and a high electron emission coefficient, which can be further improved by alloying tungsten with certain metal oxides.
Tungsten is chemically resistant. Hydrochloric, sulfuric, nitric, hydrofluoric acids, aqua regia, aqueous solution of sodium hydroxide, ammonia (up to 700° C), mercury and mercury vapor, air and oxygen (up to 400° C), water, hydrogen, nitrogen, carbon monoxide (up to 800° C), hydrogen chloride (up to 600° C) have no effect on tungsten. Ammonia mixed with hydrogen peroxide, liquid and boiling sulfur, chlorine (over 250 ° C), hydrogen sulfide at red heat, hot aqua regia, a mixture of hydrofluoric and nitric acids, melts of nitrate, nitrite, potassium chlorate, lead dioxide react with tungsten. , sodium nitrite, hot nitric acid, fluorine, bromine, iodine. Tungsten carbide is formed by the interaction of carbon with tungsten at temperatures above 1400 ° C, oxide - by interaction with water vapor and sulfur dioxide (at red heat), carbon dioxide (above 1200 ° C), oxides of aluminum, magnesium and thorium.
Prices at reception points
To form average prices, consider the cost of receiving tungsten scrap at three types of enterprises :
- those that accept scrap with a total weight of several tens of kilograms ;
- enterprises purchasing metal from 0.5 kilograms;
- places where you can donate tungsten scrap up to 0.5 kilograms.
Large parties
Mosvtormetal
This Moscow enterprise accepts various types of scrap raw materials, including tungsten. The company's website contains information that scrap is purchased from 50 kilograms , while the price for one kilogram of pure metal ranges from 900 to 950 rubles , depending on the condition in which the scrap was delivered to the enterprise.
Resistance calculation
Electrical resistance is of particular importance in situations where the wire is used as a winding for transformers and generators. After all, if the resistance is too high, then in the event of an emergency, the winding may ignite, which can lead to catastrophic consequences.
Resistance formula
To accurately calculate resistance, use the following formula: R = (P x L)/S. It deciphers like this:
- R is the total resistance. We need to find this parameter as a result of calculations (units of measurement - Ohm).
- P is the resistivity of the material. This indicator is a physical constant, and it depends on the type of chemical element. For copper the constant P will be equal to 0.0175 (units - (Ohm x mm x mm)/m).
- L is the total length in meters. The larger it is, the higher the resistance of the conductor will be.
- S is the cross-sectional area in square millimeters. This parameter also affects the final resistance - the smaller it is, the higher the resistance will be.
Please note that the parameter S is usually indicated in the technical documentation, but instead of the cross-sectional area, sometimes only the cross-sectional diameter of the wire is indicated. In this case, it is necessary to calculate the area using the formula: S = (Pi xdxd)/4
This formula is deciphered as follows:
- Pi is a mathematical constant that is approximately equal to 3.14.
- d is the cross-sectional diameter of the conductor in millimeters.
As a result, the resistance of copper wire is measured using two formulas: R = (P x L)/S = (4 x P x L)/(Pi xdxd).
Examples of problems
Let's try to solve a few simple problems:
- Task 1. Determine the resistance of a wire whose length is 100 meters and cross-sectional area is 5 square millimeters. In our problem, the area parameter is known, so we will use the first formula R = (P x L)/S. Let's substitute our values: R = (0.0175 x 100)/5 = 0.35 Ohm.
- Task 2. Determine the resistance of a wire whose length is 500 meters and cross-sectional diameter is 2 millimeters. In this problem the diameter is known, so we will use the second formula R = (4 x P x L)/(Pi xdxd). Let's substitute our values: R = (4 x 0.0175 x 500)/(3.14 x 2 x 2) = 2.78 Ohm.
Nichrome from a soldering iron
Disassemble the soldering iron and find the same nichrome wire.
We actually found one, but it was very thin. You can find out the cross-section of the wire by winding 10 turns of thread tightly pressed together around a pencil. Our turns took 1 mm. This means that the cross-section of our thread is 0.1 mm. You can buy a lot of such cheap soldering irons, but the nichrome spiral in them may differ in length. Disassembling a household soldering iron to obtain nichrome is a really working scheme. But what to do if you don’t have a soldering iron?
A similar spiral is found in hair dryers. Different models have nichrome threads of different diameters, but they are definitely there. Nichrome can be found in a heater built on the principle of a fan. This option will be slightly more expensive than the previous one.
And finally, the most difficult way is to get this metal out of an electric stove with an open spiral. Today we told you that nichrome winding is not such an expensive undertaking. If you decide to find a thread in a soldering iron, then it can reach 2.5 m. This will turn out to be far from 1 winding.
Areas of application
Tungsten wire is used in various fields of production and the national economy. It is used for the manufacture of spirals and spring elements intended for incandescent light bulbs.
Tungsten-rhenium variety (TRN) is used for the production of traverses.
Tungsten is a refractory metal, so wire products based on it are indispensable when creating resistance elements in heating devices. It is contained in thermoelectric converters and loop heaters.
The manufacturing process of tungsten rolled metal is quite complex, involving powder metallurgy techniques. It is very popular in the electrical and radio engineering industries. It is actively used in the creation of LCD television screens. The most popular wire products are tungsten anhydride and obtained from salts of this metal.
It is used to make parts of X-ray equipment, which during operation are subject to vibrations and strong heating. Meshes and filter mechanisms based on it are used in the chemical industry.