Definition of corrosion
Metal materials under chemical or electrochemical influence of the environment are subject to destruction, which is called corrosion.
Corrosion of metals is caused by redox reactions, as a result of which metals become oxidized and lose their properties, which renders metal materials unusable.
There are 3 signs that characterize corrosion:
- Corrosion is, from a chemical point of view, a redox process.
- Corrosion is a spontaneous process that occurs due to the instability of the thermodynamic system metal - environmental components.
- Corrosion is a process that develops mainly on the surface of the metal. However, it is possible that corrosion can penetrate deep into the metal.
Why does metal corrode?
The life of a modern person cannot be imagined without metals. They surround us everywhere - these are household appliances in our homes, and the vehicles we use to get to home or work, and smartphones, without which many of us cannot imagine life. Almost everything that surrounds us consists of metals, but, unfortunately, like everything in this world, they are not eternal and under the influence of the external environment they are destroyed - they corrode.
Why is corrosion “beneficial” for metals? The fact is that most of them exist in nature in a chemically bound state, for example, in the form of oxides (corundum) or sulfides (pyrite). In their pure form, almost all metals are unstable and considerable energy must be expended to separate them from their compounds. The reverse process, when metals go into a bound state, is always thermodynamically more favorable. Therefore, it occurs spontaneously, and metals, whenever possible, tend to react with their environment and transform into a more stable form. An illustration of this is shown in Figure 1.
Figure 1 – Scheme of metal recovery from ores followed by corrosion (oxidation). E – conditional energy level. Corrosion leads to huge economic costs, and its consequences are global environmental disasters. The loss of metal stock from corrosion is about 12% per year.
In addition to direct losses, there are also indirect losses caused by corrosion:
- due to equipment downtime caused by accidents;
- due to reduced equipment capacity;
- due to a decrease in product quality;
- to eliminate the consequences of the accident;
- for equipment repair;
- for further protection against corrosion.
Types of metal corrosion
The most common types of metal corrosion :
- Uniform – covers the entire surface evenly
- Uneven
- Electoral
- Local stains – individual areas of the surface are corroded
- Ulcerative (or pitting)
- Spot
- Intercrystalline - spreads along the boundaries of a metal crystal
- Cracking
- Subsurface
Main types of metal corrosion
From the point of view of the mechanism of the corrosion process, two main types of corrosion can be distinguished: chemical and electrochemical.
Order metal galvanization from Tochinvest Zinc
Each client when contacting our company receives the following benefits:
- The company has been operating since 2007 and has at its disposal 3 production workshops for hot-dip galvanizing.
- The capacity of the enterprise with many years of experience is 120,000 tons per year.
- Prompt execution of work, regardless of complexity and volume.
- Our company is the owner of the deepest hot-dip galvanizing bath in the Central Federal District - the depth is 3.43 m.
- We apply zinc coating to various types of metal structures, including large ones.
- Specialists perform coating application using hot-dip galvanizing technology in accordance with the requirements of GOST 307-89.
The work is carried out using modern equipment from the Czech company EKOMOR and the German-Austrian company KVK KOERNER.
Return to articles Share article
Electrochemical corrosion of metals
Electrochemical corrosion of metals is the process of destruction of metals in the environment of various electrolytes, which is accompanied by the appearance of an electric current inside the system.
With this type of corrosion, an atom is removed from the crystal lattice as a result of two coupled processes :
- Anodic - metal in the form of ions goes into solution.
- Cathode – the electrons formed during the anodic process are bound by a depolarizer (the substance is an oxidizing agent).
The process of removing electrons from the cathode sites is called depolarization , and substances that promote removal are called depolarizers.
The most common corrosion of metals is with hydrogen and oxygen depolarization .
Hydrogen depolarization
Hydrogen depolarization is carried out at the cathode during electrochemical corrosion in an acidic environment :
2H++2e— = H2 discharge of hydrogen ions
2H3O++2e— = H2 + 2H2O
Oxygen depolarization
Oxygen depolarization is carried out at the cathode during electrochemical corrosion in a neutral environment :
O2 + 4H++4e— = H2O reduction of dissolved oxygen
O2 + 2H2O + 4e— = 4OH—
All metals, in their relation to electrochemical corrosion, can be divided into 4 groups, which are determined by the values of their standard electrode potentials:
- Active metals (high thermodynamic instability) are all metals in the range of alkali metals - cadmium (E0 = -0.4 V). Their corrosion is possible even in neutral aqueous environments in which there is no oxygen or other oxidizing agents.
- Metals of medium activity (thermodynamic instability) are located between cadmium and hydrogen (E0 = 0.0 V). In neutral environments, in the absence of oxygen, they do not corrode, but are subject to corrosion in acidic environments.
- Low-active metals (intermediate thermodynamic stability) - are located between hydrogen and rhodium (E0 = +0.8 V). They are resistant to corrosion in neutral and acidic environments in which there is no oxygen or other oxidizing agents.
- Noble metals (high thermodynamic stability) - gold, platinum, iridium, palladium. They can be subject to corrosion only in acidic environments in the presence of strong oxidizing agents.
Types of electrochemical corrosion
Electrochemical corrosion can occur in various environments. Depending on the nature of the environment, the following types of electrochemical corrosion are distinguished:
- Corrosion in electrolyte solutions - in solutions of acids, bases, salts, in natural water.
- Atmospheric corrosion - in atmospheric conditions and in any humid gas environment. This is the most common type of corrosion.
For example, when iron interacts with environmental components, some of its sections serve as the anode, where iron oxidation occurs, and others serve as the cathode, where oxygen reduction occurs:
A: Fe – 2e— = Fe2+
K: O2 + 4H+ + 4e— = 2H2O
The cathode is the surface where the oxygen flow is greater.
- Soil corrosion - depending on the composition of the soil, as well as its aeration, corrosion can occur more or less intensely. Acidic soils are the most aggressive, while sandy soils are the least.
- Aeration corrosion occurs when there is uneven access of air to different parts of the material.
- Marine corrosion - occurs in sea water due to the presence of dissolved salts, gases and organic substances in it .
- Biocorrosion - occurs as a result of the activity of bacteria and other organisms that produce gases such as CO2, H2S, etc., which contribute to metal corrosion.
- Electrocorrosion - occurs under the influence of stray currents in underground structures, as a result of the work of electric railways, tram lines and other units.
Classification of corrosion processes according to the nature of corrosion destruction.
4.1 Contact corrosion.
Contact corrosion occurs when different metals come into contact in the presence of an electrolyte or moist air. In the resulting galvanic couple, the metal with a more electronegative potential becomes the anode and is destroyed first, while the more electropositive metal becomes the cathode.
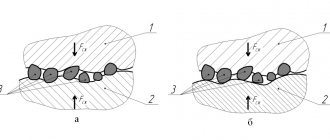
As an example, consider iron (Figure 4). Zinc, aluminum and cadmium (the latter in a salt environment) are anodes for steel, which means they will be oxidized first, while tin, chromium, copper, lead, nickel are cathodes, which means iron will be subject to deep local corrosion, an example is shown in the figure.
Figure 4 – Examples of contact corrosion on steel.
4.2 Crevice corrosion.
Crevice corrosion
is corrosion that occurs when part of the metal is isolated from the main area by non-metallic material (rubber, wood, plastic, etc.). An example of such corrosion can be observed in pipes at the point of contact with the seal (Figure 5). The formation of a cavity under the seal causes leaks in the pipes. In the presence of such irregularities, the corrosive liquid stagnates in the crack, where rapid corrosion of the metal occurs.
The cause of crevice corrosion is a reduced concentration of oxidizing agents in the gaps compared to the volume of the solution and a slow removal of corrosion products. As a result of their accumulation, the pH of the solution in the gap changes, which also accelerates corrosion.
The metal in the gap and the metal on the open surface form a macropair:
Me - 2e = Me2+ (inside the gap) 0.5O2 + H2O + 2e = 2OH- (on the metal surface)
Since the exposed surface area is much larger than that inside the crevice, the corrosion current density inside the crevice is extremely high.
Figure 5 – Oxygen concentration cell under the seal. As corrosion progresses, excess positive charge accumulates inside the crack. OH- ions rush into the gap to neutralize this charge. As a result, metal hydroxide is deposited on the inner surface of the gap, which further reduces the effective area of the anode.
4.3 Pitting corrosion.
Pitting
are called deep lesions (pinpoint ulcers) on the metal surface (Figure 6). Pitting corrosion, due to its localization and low visibility, is one of the most dangerous types of corrosion damage. Pitting corrosion should not be confused with pitting on nickel coatings.
Since the passivating layer on the metal surface is not a homogeneous system, corrosion occurs due to the presence of anodic and cathodic areas on the surface. In the resulting galvanic couple, the anode is the pitting, and the cathode is the rest of the surface. At the anode, electrons are released, which reduce oxygen on the cathode passivated part of the surface.
Figure 6 – Types of pitting: a – open with a protective layer on the surrounding surface; b – closed, without a surrounding protective layer; c – closed, with a surrounding protective layer. 1 – metal; 2 – solution; 3 – protective layer; 4 – porous corrosion products; 5 – cover over the pitting; 6 – holes in the cover. The development of pitting is facilitated by various types of defects on the surface of the passive film, for example, scratches, chips, pores, and foreign inclusions. Also, for pitting to occur, it is necessary that the solution simultaneously contain pitting corrosion activators (Cl-, Br-, J-, CN-) and metal passivators (OH-, SO42-, NO3-, ClO4-).
4.4 Intergranular corrosion.
Intercrystalline corrosion
arises due to the difference in potentials at the grain boundary and in its matrix (Figure 7).
In air, a carbide phase forms at the grain boundary, which shifts the potential to more electronegative values. Thus, the grain boundary is the anode with respect to their matrix.
This type of corrosion is most dangerous for alloys, since in the place where the more electronegative metal accumulates, an anode will form, and the main one - a cathode. For example, for stainless steels containing chromium, the chromium content near the grain boundaries is lower than on the rest of the surface, which makes them less passivated. As a result, such places become anodes in relation to the grain matrix.
Figure 7 – Intercrystalline corrosion of stainless steel: 1 – cathode; 2 – anode; 3 – carbide phase; 4 – chromium-depleted zone; 5 – grain boundary.
4.5 Fretting corrosion.
Fretting corrosion
occurs between two surfaces that are in continuous contact with each other and undergo small vibrations. The surfaces are never separated from each other, so fragments of corrosion products accumulate at points of mechanical contact.
This corrosion occurs during minor vibrations, cyclic or reciprocating movements with small amplitudes and speeds. Bolts, rivets, hinges, couplings, valves, engine parts, etc. are subject to this corrosion.
4.6 Stress corrosion cracking.
Stress corrosion cracking occurs when a metal product is subjected to tension in a corrosive environment. Then, even at stresses below the fracture stress, cracking occurs, ultimately leading to the destruction of the structure or product.
Corrosion occurs on stretched areas of the metal, since they turn out to be anodes in relation to the unstretched part. This phenomenon is observed on any metals and alloys, as well as in any environment.
4.7 Corrosion fatigue.
Corrosion fatigue occurs due to simultaneous exposure to an aggressive environment and mechanical load.
Corrosion fatigue often causes “unexpected” failure of metal parts, so if a part in a corrosive environment is subject to continuous vibration, its failure will occur at a stress well below the endurance limit.
Methods of protection against metal corrosion
The main method of protecting metal from corrosion is the creation of protective coatings - metallic, non-metallic or chemical.
Metal coatings
A metal coating is applied to the metal that needs to be protected from corrosion with a layer of another metal that is resistant to corrosion under the same conditions. If the metal coating is made of a metal with a more negative potential (more active) than the one being protected, then it is called an anodic coating . If the metal coating is made of a metal with a more positive potential (less active) than the one being protected, then it is called a cathodic coating .
For example, when applying a layer of zinc to iron, if the integrity of the coating is compromised, the zinc acts as an anode and will be destroyed, while the iron is protected until all the zinc is used up. The zinc coating in this case is anodic .
The cathode coating to protect the iron may, for example, be copper or nickel. If the integrity of such a coating is violated, the protected metal is destroyed.
Non-metallic coatings
Such coatings can be inorganic (cement mortar, glassy mass) and organic (high molecular weight compounds, varnishes, paints, bitumen).
Chemical coatings
In this case, the protected metal is subjected to chemical treatment in order to form a corrosion-resistant film of its compound on the surface. These include:
oxidation – production of stable oxide films (Al2O3, ZnO, etc.);
phosphating – obtaining a protective film of phosphates (Fe3(PO4)2, Mn3(PO4)2);
nitriding – the surface of the metal (steel) is saturated with nitrogen;
steel bluing - the metal surface interacts with organic substances;
carburization – obtaining on the surface of a metal its connection with carbon.
Changes in the composition of technical metal and corrosive environment
Changing the composition of the technical metal also helps to increase the metal's resistance to corrosion. In this case, compounds are introduced into the metal that increase its corrosion resistance.
Changing the composition of the corrosive environment (introducing corrosion inhibitors or removing impurities from the environment) is also a means of protecting the metal from corrosion.
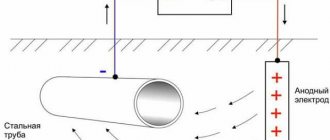
Electrochemical protection
Electrochemical protection is based on connecting the protected structure to the cathode of an external direct current source, as a result of which it becomes the cathode. The anode is scrap metal, which, when destroyed, protects the structure from corrosion.
Protective protection - one of the types of electrochemical protection - is as follows.
Plates of a more active metal, called protector . The protector - a metal with a more negative potential - is the anode, and the protected structure is the cathode. The connection of the protector and the protected structure with a current conductor leads to the destruction of the protector.
Examples of problems with solutions for determining the protective properties of oxide films, determining the corrosion resistance of metals, as well as equations for reactions occurring during electrochemical corrosion of metals are given in the section Problems for the section Corrosion of metals
Categories Corrosion of metals, GENERAL CHEMISTRY
Chemical
Chemical corrosion refers to the gradual destruction of a metal surface due to the reaction of the surface with substances in the external environment. It occurs as a result of metal oxidation with acids to form oxides.
The high-temperature option involves exposing the metal to dry gases. All metals in dry air are covered with a very thin (2...10 microns) layer of oxides. This layer is formed at very high temperatures, when the reaction with oxygen in the air occurs without any restrictions. At room temperature the reaction stops because the oxide film becomes too thin. In the case of, for example, aluminum, such a film consisting of Al2O3 oxide effectively protects the surface of aluminum cookware, since the corrosion resistance of pure aluminum is low.
Chemical corrosion begins at a place where the metal is under pressure and isolated from air circulation. This encourages metal ions to dissolve in a humid environment, which ultimately speeds up the reaction between them and water. The reaction produces hydrous oxides (known as rust when reacting with iron) and free ions.
Chemical corrosion in non-electrolyte liquids
Although gas corrosion is considered the most common, metal damage upon contact with various electrolyte liquids should also not be discounted. The greatest danger comes from contact of the material with substances that can conduct electricity.
They are divided into two large groups - organic and inorganic. There are many electrolytes that pose a great danger to metal - from molten sulfur and benzene to liquid bromine, alcohol, kerosene, oil and others.
The purity of the electrolyte is of great importance during the course of a chemical reaction. When it is completely clean, there is no interaction. But as soon as a small amount of impurities gets into the composition, the reaction begins to develop especially rapidly.
Another additional risk factor is the presence of moisture. Then the threat of electrochemical corrosion is also added to the danger of chemical corrosion.
Stages of corrosion in non-electrolyte liquids
If we consider the entire process in more detail and analyze what affects the rate of chemical corrosion, we can distinguish several stages of its occurrence:
- Contact of the oxidizing agent with the surface of the material.
- Starting the process of chemisorption of the reagent on the surface.
- The reaction of the metal and the oxidizing agent, the formation of an oxide film.
Environmental conditions, the composition of the alloy and the electrolyte itself can affect the occurrence of several basic processes. These include such as desorption of oxides with metal and diffusion of oxides into a non-electrolyte. But both processes may also not be observed.
To prevent corrosion from starting in electrolyte liquids, care should be taken to apply special protective compounds to the surface. It is important that throughout the entire period of use of the product they fully maintain their integrity.
Types of oxide films
When chemical corrosion occurs under the influence of temperature and gas environment, three types of films can form:
- Thin. It will be impossible to notice them from the outside. They are among the least durable and can be easily erased under mechanical pressure.
- Average. Can be noticed because from the outside the metal changes color slightly.
- Thick. Clearly visible to the naked eye.
In order to prevent dangerous processes of material destruction from occurring, it is important to make the film protective.