Corrosion of pipelines is the main cause of depressurization, as a result of which cracks, ruptures and cavities appear on the surface of the pipe. And therefore, protecting pipelines from corrosion is a task not only for builders or manufacturers, but also for specialists creating projects and those who will use them.
The cause of rust and corrosion on steel tanks can be the inappropriate composition of the liquid flowing through them, the wrong combination of various metals, as well as insufficient corrosion control and poorly selected protection methods. The danger of corrosion is that it can cause pipelines to leak. Repairing pipes after damage can only be done using welding.
General provisions
Corrosion processes are the oxidation of a metal, in which its atoms change from a free state, losing their electrons, to an ionic state . An underground pipeline is subject to two types of corrosion, the nature of which is worth understanding before starting to deal with them. Therefore, I will pay a little attention to their description:
Soil
Diagram showing the effects of soil corrosion on a metal pipeline
As you probably guessed from the name and the accompanying diagram, soil corrosion occurs due to contact of steel with soil. In turn, it is divided into the following subspecies:
- Chemical _ Appears as a result of exposure to iron by gases and non-electrolytes of the liquid type. It is noteworthy that with it the material is destroyed evenly, and the formation of through holes is almost impossible, which makes this type of corrosion process the least dangerous for a highway laid underground;
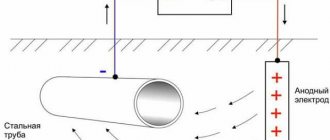
- Electrochemical . The metal acts as an electrode, and groundwater, of which there is an incredible amount in our climate zone, acts as an electrolyte. The ongoing process is very similar to the work of a galvanic couple and provokes the destruction of point areas on the surface of the pipes, which ultimately leads to their emergency condition;
The result of damage to the wall of a steel pipe by electromechanical corrosion
- Electric . It occurs due to the impact of stray currents on steel, which can “drain” from rails, substations and other electrified devices that fill modern cities. It is the most dangerous and destructive corrosion process.
We recommend: How to treat logs? Impregnations and antiseptics
Internal corrosion
Diagram showing the effects of internal corrosion on a metal pipeline
If the transported liquid has a low hydrogen index, but its content of oxygen, sulfates and chlorides, on the contrary, is high, then internal corrosion processes cannot be avoided, as a result of which:
- The level of roughness of the inner surface of the wall increases , which leads to a decrease in water permeability;
The inside of the pipeline becomes rougher due to the effects of internal corrosion
- The quality of the transported liquid deteriorates as rust gets into it;
- Over time, a through hole may appear , which can cause a pipeline rupture.
The essence of the procedure
Protective protection is based on a substance called an inhibitor. This is a metal with increased electronegative qualities. When exposed to air, the protector dissolves. As a result, the base material is preserved even if it is severely affected by corrosion.
Various types of corrosion can be easily defeated if you use cathodic electrochemical methods, which include sacrificial protection. This procedure is an ideal solution when an enterprise does not have the financial capacity or technological potential to provide complete protection against corrosion processes.
Types and properties of anti-corrosion coatings for pipelines
What is used to protect pipes from corrosion? The main treatment of pipes against corrosion can be divided into treatment of the internal surface of pipes against corrosion and protection of pipelines from external corrosion. For each surface, approximately the same materials are used, but in different proportions.
The most commonly used substances include:
- Bitumen and bitumen-polymer materials;
- Polyethylene-based materials;
- Resins;
- Primers and putties;
- Enamels;
- Paints.
The main properties of these coatings:
- Effective protection of steel pipes from corrosion;
- Relatively long service life;
- Quick and easy application;
- Possibility of application to large products and small parts;
- Economical consumption;
- Affordable price;
- Prevalence in the construction products market.
Protecting pipelines from corrosion will extend their service life
All pipeline protection methods have a large number of advantages. They are:
- increasing the strength level of pipes,
- increasing the level of resistance to the influence of an aggressive environment,
- extending the service life of pipelines of various types,
- increasing the hardness of the pipe surface both inside and outside.
Thanks to all protection methods, it is possible to ensure a long service life of all pipelines. They give them the opportunity to serve for at least ten years.
Causes
Corrosion of steel underground pipes is a phenomenon, the main reason for which can be called the reaction of electrochemical oxidation of metals from their constant interaction with moisture. As a result of such reactions, the composition of the metal changes at the ionic level, becomes covered with rust, disintegrates and simply disappears from the surface.
We recommend: How to treat wood against mold and mildew - in the basement of a wooden house
The oxidation process may be influenced by the nature of the liquid that flows through the underground heating pipeline or the properties of the environment in which it is located. It is for this reason that when choosing suitable means to combat rust, it is necessary to take into account all the features that preceded its occurrence. Otherwise, repairs by welding are inevitable.
About the features of electrochemical protection
The main cause of pipeline destruction is the result of corrosion of metal surfaces. After the formation of rust, cracks, ruptures, and cavities form, which gradually increase in size and contribute to the rupture of the pipeline. This phenomenon occurs more often near highways laid underground or in contact with groundwater.
The principle of cathodic protection is the creation of a voltage difference and the action of the two methods described above. After carrying out measuring operations directly at the location of the pipeline, it was found that the required potential to help slow down the destruction process should be 0.85V, and for underground elements this value is 0.55V.
To slow down the corrosion rate, the cathode voltage should be reduced by 0.3V. In this situation, the corrosion rate will not exceed 10 microns/year, and this will significantly extend the service life of technical devices.
One of the significant problems is the presence of stray currents in the soil. Such currents arise from the grounding of buildings, structures, rail tracks and other devices. Moreover, it is impossible to make an accurate assessment of where they may appear.
To create a destructive effect, it is enough to charge steel pipelines with a positive potential in relation to the electrolytic environment, these include pipelines laid in the ground.
In order to provide the circuit with current, it is necessary to supply an external voltage, the parameters of which will be sufficient to break through the resistance of the soil foundation.
As a rule, such sources are power lines with power ratings from 6 to 10 kW. If electric current cannot be supplied, then diesel or gas generators can be used. The installer for the protection of underground pipelines from corrosion must be familiar with the design solutions before performing work.
We recommend: How to put rubber crumbs to good use at home and in the country
Protection of underground pipelines from corrosion
Pipelines of various types are widely used in the modern world. They are almost always hidden under the ground. The process of corrosion formation on them is not one that can be avoided. It can only be delayed for a certain period of time. For this, special compounds are used that form a small protective film on the metal surface. It prevents the aggressive underground environment from affecting the structure of the pipeline.
Protection of pipelines from corrosion is aimed at stopping all oxidation processes.
Attention: It is worth noting that corrosion forms on pipes both inside and outside. Their internal part suffers from the fact that a corrosive coating appears as a result of the flow of aggressive substances through them, causing oxidative processes. The interior suffers from high soil moisture levels.
The protective film should be on both the inside and outside for obvious reasons. Only in this case can the faster appearance of a corrosive coating, which has destructive properties, be prevented.
Pipeline protection is necessary for various types of communications. Today, protective methods are used not only for water pipes that suffer from rust, but also for gas pipelines.
Protecting water pipes is necessary because they carry water to businesses and people's homes. It should be without any impurities. If the pipes are rusty, the tap fluid will have an unpleasant orange tint. This water is not suitable for human consumption. It is not even used in industrial facilities, because it can affect the properties of the products.
Table. Metal corrosion rate.
| Point | Corrosion rate | Durability group |
| 1 | <0.1 | highly resistant |
| 2 | 0.1-1.0 | persistent |
| 3 | 1.1-3.0 | reduced durability |
| 4 | 3.1-10.0 | low-resistant |
| 5 | >10.1 | unstable |
Application of anti-corrosion coating
The method of applying anti-corrosion coating depends on the selected coating material and requires an individual approach. However, there are uniform rules that apply in any case:
- The surface is prepared: cleaned of scale, rust, old protective coating, paint;
- Clean the cleaned surface;
- The surface is degreased using special compounds;
- Clean using a sand or shot blasting machine with fine sand;
- Treated with detergents to clean the deep layers of the product;
- Rinse the surface;
- Dry the surface before applying the main protective coating;
- Each layer of protective coating applied is thoroughly dried.
Anti-corrosion painting of pipes is most often used, since this material is widespread, affordable in price, easy to apply (spray or roller applied) and durable.
Equipment used for anti-corrosion treatment of pipes
Depending on the type of protective coating, special equipment is used, for example, an electric arc metallization installation (allows the application of metal coatings), plasma spraying installations, cold galvanizing installations for steel products (for paint and varnish products), spraying installations (primers and paints and varnishes) ), roller.
It is mandatory to comply with safety precautions when performing work. The specialists performing the processing must wear special protective uniforms.
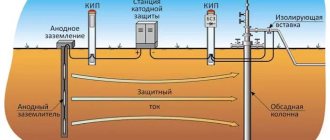
Classification of electrochemical cathodic protection techniques
This method of preventing corrosion was proposed in the 20s of the 19th century and was initially used in shipbuilding: the copper hulls of ships were sheathed with anode protectors, which significantly reduced the rate of metal corrosion.
After the effectiveness of the new technology was established, the invention began to be actively used in other areas of industry. After some time, it was recognized as one of the most effective ways to protect metals.
There are currently two main types of cathodic protection of pipelines against corrosion:
- The simplest method : an external source of electric current is supplied to a metal product that requires protection from corrosion. In this design, the part itself acquires a negative charge and becomes the cathode, while the role of the anode is performed by inert, design-independent electrodes.
- Galvanic method . The part in need of protection comes into contact with a protective (tread) plate made of metals with high values of negative electrical potential: aluminum, magnesium, zinc and their alloys. In this case, both metal elements become anodes, and the slow electrochemical destruction of the protector plate ensures that the required cathode current is maintained in the steel product. After a more or less long time, depending on the parameters of the plate, it dissolves completely.
Characteristics of the first method
This method of ECP of pipelines, due to its simplicity, is the most common. It is used to protect large structures and elements, in particular, underground and above-ground pipelines.
The technique helps to resist:
- pitting corrosion;
- corrosion due to the presence of stray currents in the area where the element is located;
- corrosion of intercrystal type stainless steel;
- cracking of brass elements due to increased stress.
Characteristics of the second method
This technology, unlike the first one, is intended, among other things, to protect small-sized products. The technique is most popular in the USA, while it is rarely used in the Russian Federation. The reason is that to carry out galvanic electrochemical protection of pipelines, it is necessary to have an insulating coating on the product, and in Russia main pipelines are not treated in this way.