Definition of corrosion
Metal materials under chemical or electrochemical influence of the environment are subject to destruction, which is called corrosion.
Corrosion of metals is caused by redox reactions, as a result of which metals become oxidized and lose their properties, which renders metal materials unusable.
There are 3 signs that characterize corrosion:
- Corrosion is, from a chemical point of view, a redox process.
- Corrosion is a spontaneous process that occurs due to the instability of the thermodynamic system metal - environmental components.
- Corrosion is a process that develops mainly on the surface of the metal. However, it is possible that corrosion can penetrate deep into the metal.
NATIONAL ECONOMIC IMPORTANCE OF COMBATING CORROSION
Losses from corrosion can be divided into direct and indirect. Direct losses are the cost of replaced products, the cost of protective measures and irretrievable loss of metal due to corrosion. According to experts, on a global scale these currently account for about 10...15% of steel production. Indirect – product losses as a result of leaks, decreased unit performance, contamination of the target product with corrosion products, etc.
A significant part of the power of ferrous metallurgy enterprises is spent on replenishing metal losses due to corrosion. However, this does not fully reflect the actual damage associated with the failure of metal products. Significant losses are caused by equipment failures, downtime, losses and waste in metalworking, violations of product quality and, ultimately, an increase in its cost and a decrease in labor productivity. Therefore, saving metal, improving the quality of raw materials and metal products, reducing corrosion losses is an indispensable condition for increasing production efficiency and product quality, which must be ensured on a national scale.
Types of metal corrosion
The most common types of metal corrosion :
- Uniform – covers the entire surface evenly
- Uneven
- Electoral
- Local stains – individual areas of the surface are corroded
- Ulcerative (or pitting)
- Spot
- Intercrystalline - spreads along the boundaries of a metal crystal
- Cracking
- Subsurface
Main types of metal corrosion
From the point of view of the mechanism of the corrosion process, two main types of corrosion can be distinguished: chemical and electrochemical.
Electrochemical corrosion of metals
Electrochemical corrosion of metals is the process of destruction of metals in the environment of various electrolytes, which is accompanied by the appearance of an electric current inside the system.
With this type of corrosion, an atom is removed from the crystal lattice as a result of two coupled processes :
- Anodic - metal in the form of ions goes into solution.
- Cathode – the electrons formed during the anodic process are bound by a depolarizer (the substance is an oxidizing agent).
The process of removing electrons from the cathode sites is called depolarization , and substances that promote removal are called depolarizers.
The most common corrosion of metals is with hydrogen and oxygen depolarization .
Hydrogen depolarization
Hydrogen depolarization is carried out at the cathode during electrochemical corrosion in an acidic environment :
2H++2e— = H2 discharge of hydrogen ions
2H3O++2e— = H2 + 2H2O
Oxygen depolarization
Oxygen depolarization is carried out at the cathode during electrochemical corrosion in a neutral environment :
O2 + 4H++4e— = H2O reduction of dissolved oxygen
O2 + 2H2O + 4e— = 4OH—
All metals, in their relation to electrochemical corrosion, can be divided into 4 groups, which are determined by the values of their standard electrode potentials:
- Active metals (high thermodynamic instability) are all metals in the range of alkali metals - cadmium (E0 = -0.4 V). Their corrosion is possible even in neutral aqueous environments in which there is no oxygen or other oxidizing agents.
- Metals of medium activity (thermodynamic instability) are located between cadmium and hydrogen (E0 = 0.0 V). In neutral environments, in the absence of oxygen, they do not corrode, but are subject to corrosion in acidic environments.
- Low-active metals (intermediate thermodynamic stability) - are located between hydrogen and rhodium (E0 = +0.8 V). They are resistant to corrosion in neutral and acidic environments in which there is no oxygen or other oxidizing agents.
- Noble metals (high thermodynamic stability) - gold, platinum, iridium, palladium. They can be subject to corrosion only in acidic environments in the presence of strong oxidizing agents.
Types of electrochemical corrosion
Electrochemical corrosion can occur in various environments. Depending on the nature of the environment, the following types of electrochemical corrosion are distinguished:
- Corrosion in electrolyte solutions - in solutions of acids, bases, salts, in natural water.
- Atmospheric corrosion - in atmospheric conditions and in any humid gas environment. This is the most common type of corrosion.
For example, when iron interacts with environmental components, some of its sections serve as the anode, where iron oxidation occurs, and others serve as the cathode, where oxygen reduction occurs:
A: Fe – 2e— = Fe2+
K: O2 + 4H+ + 4e— = 2H2O
The cathode is the surface where the oxygen flow is greater.
- Soil corrosion - depending on the composition of the soil, as well as its aeration, corrosion can occur more or less intensely. Acidic soils are the most aggressive, while sandy soils are the least.
- Aeration corrosion occurs when there is uneven access of air to different parts of the material.
- Marine corrosion - occurs in sea water due to the presence of dissolved salts, gases and organic substances in it .
- Biocorrosion - occurs as a result of the activity of bacteria and other organisms that produce gases such as CO2, H2S, etc., which contribute to metal corrosion.
- Electrocorrosion - occurs under the influence of stray currents in underground structures, as a result of the work of electric railways, tram lines and other units.
Popular rust removers
Currently, there are dozens of different rust converters in auto stores, and their range may vary in different regions of the country. Therefore, it makes no sense to give recommendations regarding the purchase of this or that product. But we will still give as an example several names of popular compounds that are common among car owners. So:
Popular remedy "Tsinkar"
- "Tsinkar";
- "Movil";
- Hi-Gear line of rust converters;
- "Chain mail";
- Sonax;
- "SF-1";
- Runway;
- Permatex;
- Bitumast;
- "Phosphomet".
It must be remembered that with the help of any converter you can fight rust, the layer of which does not exceed 0.1 mm. In addition, the active components only fight stubborn rust. It is better to remove its loose component mechanically (using sandpaper, a knife, a metal brush, sandblasting, and so on).
When working with converters, follow safety regulations. It is advisable to work with rubber gloves and prevent liquid from coming into contact with exposed skin.
The choice of one product or another should be based on the range, its composition, and price. Fortunately, they are inexpensive, so if the purchased product turns out to be ineffective, you can always buy another one.
Methods of protection against metal corrosion
The main method of protecting metal from corrosion is the creation of protective coatings - metallic, non-metallic or chemical.
Metal coatings
A metal coating is applied to the metal that needs to be protected from corrosion with a layer of another metal that is resistant to corrosion under the same conditions. If the metal coating is made of a metal with a more negative potential (more active) than the one being protected, then it is called an anodic coating . If the metal coating is made of a metal with a more positive potential (less active) than the one being protected, then it is called a cathodic coating .
For example, when applying a layer of zinc to iron, if the integrity of the coating is compromised, the zinc acts as an anode and will be destroyed, while the iron is protected until all the zinc is used up. The zinc coating in this case is anodic .
The cathode coating to protect the iron may, for example, be copper or nickel. If the integrity of such a coating is violated, the protected metal is destroyed.
Non-metallic coatings
Such coatings can be inorganic (cement mortar, glassy mass) and organic (high molecular weight compounds, varnishes, paints, bitumen).
Chemical coatings
In this case, the protected metal is subjected to chemical treatment in order to form a corrosion-resistant film of its compound on the surface. These include:
oxidation – production of stable oxide films (Al2O3, ZnO, etc.);
phosphating – obtaining a protective film of phosphates (Fe3(PO4)2, Mn3(PO4)2);
nitriding – the surface of the metal (steel) is saturated with nitrogen;
steel bluing - the metal surface interacts with organic substances;
carburization – obtaining on the surface of a metal its connection with carbon.
Changes in the composition of technical metal and corrosive environment
Changing the composition of the technical metal also helps to increase the metal's resistance to corrosion. In this case, compounds are introduced into the metal that increase its corrosion resistance.
Changing the composition of the corrosive environment (introducing corrosion inhibitors or removing impurities from the environment) is also a means of protecting the metal from corrosion.
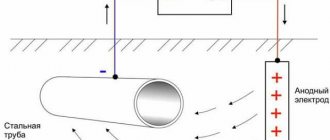
Electrochemical protection
Electrochemical protection is based on connecting the protected structure to the cathode of an external direct current source, as a result of which it becomes the cathode. The anode is scrap metal, which, when destroyed, protects the structure from corrosion.
Protective protection - one of the types of electrochemical protection - is as follows.
Plates of a more active metal, called protector . The protector - a metal with a more negative potential - is the anode, and the protected structure is the cathode. The connection of the protector and the protected structure with a current conductor leads to the destruction of the protector.
Examples of problems with solutions for determining the protective properties of oxide films, determining the corrosion resistance of metals, as well as equations for reactions occurring during electrochemical corrosion of metals are given in the section Problems for the section Corrosion of metals
Categories Corrosion of metals, GENERAL CHEMISTRY
Video description
How to remove rust using Silit, watch this video.
Some home craftsmen use Coca-Cola or Pepsi to get rid of corrosion. These drinks contain phosphoric acid, which easily removes small rust stains. Wipe metal surfaces with liquids or immerse parts in them. Then rinse under running water.
Pepsi can easily cope with rust in small areas Source yandex.net
A mixture based on paraffin and kerosene is also popular. Take these products in equal proportions and mix. Apply to the surface to be treated in several thin layers with a sponge or brush. Leave it for at least a day. After the specified time, remove the mixture along with the rust with a rag, rinse with water and remove moisture with a rag or sponge. It is best to use this method for tools. Do not forget about a mask, gloves and safety glasses when processing with a kerosene-paraffin mixture.