Electrochemical corrosion of metals has been one of the pressing problems of humanity since the moment metal products began to be used in the production of various objects necessary for humans. The problem of protecting metals from corrosion has always been acute, because under the influence of destructive oxidation processes, objects lost functionality, became deformed and became unusable, and it was necessary to look for ways to protect them.
When chemistry emerged as a separate science, and the use of metals began to acquire widespread industrial significance, humanity began to explore these processes and look for ways to combat destruction from external influences.
What is corrosion
The process of destruction of the top layer of a metal material under the influence of external influences is called corrosion in the broad sense.
The term corrosion in this case is only a characteristic of the fact that a metal surface enters into a chemical reaction and loses its original properties under its influence.
There are 4 main signs by which you can determine that this process exists:
- a process that develops on the surface and eventually penetrates into the metal product;
- the reaction occurs spontaneously because the stability of the thermodynamic balance between the environment and the system of atoms in the alloy or monolith is disrupted;
- chemistry perceives this process not simply as a destruction reaction, but as a reduction and oxidation reaction: when entering into a reaction, some atoms replace others;
- the properties and characteristics of the metal undergo significant changes during such a reaction, or are lost where it occurs.
Corrosion protection methods
Rust and other corrosive effects can lead to safety issues and compromise the integrity of production equipment and supplies. Even routine maintenance to remove and remove rust increases operating costs. A number of methods have been developed that can be used to minimize corrosion.
Types of corrosion
Depending on the type of metal and the redox reaction occurring with it, corrosion can be:
- uniform or uneven;
- local and point (individual areas for some reason reacted, while others did not);
- ulcerative, also known as pitting;
- subsurface;
- cracking;
- intercrystalline, occurring along the boundaries of a metal crystal.
Also, depending on what external factors affect the surface, corrosion can be chemical or electrochemical. Chemical corrosion occurs as a result of certain reactions under the influence of chemical interactions, but without the participation of electric current, and can even be inherent in oil and gas. Electrochemical is distinguished by certain processes; it is more complex than chemical.
On video: metal corrosion.
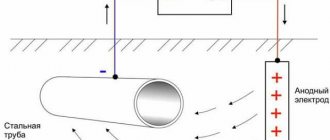
Electrochemical
To simulate the process, it is necessary to consider an iron plate coated with any electrically conductive coating, for example, oxide scale, which was formed during high-temperature processing. When the plate is immersed in a solution of sodium chloride, it is discovered that if the integrity of the scale is damaged, the rusting of the iron will occur much faster in this place. Electrochemical corrosion most reliably explains the rusting of iron under aerobic conditions.
The theory of electrochemical corrosion assumes the presence of additional chemical reactions:
- Fe → Fe ++ + 2e−, - anodic reaction;
- 2e− + O + H2O → 2OH− - cathodic reaction.
When metal ions dissolve, their charge is balanced by chloride ions, which migrate into the area of attack, attracted by the resulting positive ions. Ferric chloride dissolves in water, but this does not prevent further corrosion, since the ferric chloride solution is very acidic due to hydrolysis. As Fe++ ions are removed from this site, they encounter hydroxyl ions, which are either naturally present in the water or formed through a cathodic reaction. The result is the formation and precipitation of iron hydroxide Fe (OH)2. Further, in the presence of dissolved oxygen, it is quickly oxidized to iron oxyhydroxide FeOOH.
Thus, during electrochemical corrosion, three reactions occur, and in three different places. Anodic occurs in areas of metal loss, cathodic - where oxygen dissolved in water can accept electrons, and solid scale itself is formed in places of mechanical damage on the surface of the product.
Recently, another type of corrosion has been identified - mechanochemical, which occurs as a result of the dynamic interaction of contacting environmental elements under conditions of high contact pressures.
Causes and signs of electrochemical corrosion
Electrochemical corrosion differs from chemical corrosion in that the destruction process takes place in a system of electrolytes, which causes an electric current to arise within this system. Two coupled processes, anodic and cathodic, lead to the removal of unstable atoms from the crystal lattice of the metal. During the anodic process, the ions go into solution, and the electrons from the anodic process fall into the trap of the oxidizing substance and are bound by the depolarizer.
Thus, depolarization is the removal of free electrons from the cathode sites, and the depolarizer is the substance that is responsible for this process. The main reactions occur with the participation of hydrogen and oxygen as depolarizers.
There are many examples of electrochemical corrosion of various types that affect metal surfaces in nature and are influenced by various conditions. Hydrogen works in an acidic environment, and oxygen in a neutral environment.
Almost all metals are subject to electrochemical corrosion, and on this basis they are divided into 4 groups, and the value of their electrode potential is determined:
- active ones corrode even in an environment where there are no oxidizing agents;
- moderately active ones enter into an oxidation reaction in an acidic environment;
- low-active ones do not react in the absence of oxidizing agents in both neutral and acidic environments;
- do not react - high stability (noble metals, palladium, gold, platinum, iridium).
The most common type of electrochemical corrosion is atmospheric.
But the same reaction can also occur in water, in solutions of bases, salts and acids. In the highly specialized distinction of atmospheric corrosion, a distinction is made between soil and aeration, marine and biological (occurring under the influence of bacteria).
There is even electrical corrosion, which occurs under the influence of electric current, and is the result of stray currents that arise where electric current is used by a person to carry out a certain activity.
The homogeneous metal surface is destroyed due to thermodynamic instability to the environment. And heterogeneous - due to the composition of the crystal lattice, in which atoms of one metal are held together more tightly than atoms of foreign inclusions. These reactions differ in the rate of ionization of ions and the reduction of oxidative components of the environment.
The destruction of metal surfaces during electrochemical corrosion consists of the simultaneous occurrence of two processes: anodic and cathodic, and the differences between the processes are that dissolution occurs on the anodes, which are in contact with the environment through many microelectrodes, which are part of the surface of any metal and are closed to myself.
Typical examples of electrochemical corrosion can be considered the occurrence of corrosion processes on the bottoms of sea vessels or in the atmosphere on metal structures.
Classification of corrosion processes according to the conditions under which corrosion occurs.
- Gas corrosion occurs in the gas phase with a minimal amount of moisture. This corrosion occurs when metals come into contact with aggressive gases (halogens, oxygen, sulfur oxide).
- Atmospheric corrosion occurs in an atmosphere of air or other moist gas.
- Liquid corrosion is corrosion that occurs in various types of liquids.
- Underground corrosion is metal corrosion that occurs as a result of inhomogeneities in the soil.
- Corrosion under cryptoclimate conditions occurs in confined spaces.
- Radiation corrosion is caused by exposure to radiation.
- Marine corrosion occurs due to the depassivating properties of chlorine ions.
- Structural corrosion is associated with the structural heterogeneity of metals.
- Corrosion caused by stray currents.
The need for anti-corrosion protection
Protecting metal from influences that have a destructive effect on its surface is one of the main tasks facing those people who work with mechanisms, units and machines, ships and construction processes.
Any metal, except noble ones, to one degree or another, is exposed to destructive processes.
The more actively a device or part is used, the more likely it is to be subject to the destructive effects of atmospheric conditions and liquids that are encountered during operation. Many branches of science and industrial production are working to protect metal from corrosion, but the main methods remain unchanged and consist of creating protective coatings:
- metal;
- non-metallic;
- chemical
Non-metallic coatings are created using organic and inorganic compounds; their operating principle is quite effective and differs from other types of protection. To create non-metallic protection in industrial and construction production, paint and varnish compositions, concrete and bitumen and high-molecular compounds are used, especially actively adopted in recent years, when polymer chemistry has reached great heights.
Chemistry has contributed to the creation of protective coatings using methods:
- oxidation (creating a protective film on metal using oxide films);
- phosphating (phosphate films);
- nitriding (saturation of the steel surface with nitrogen);
- cementation (combination with carbon);
- bluing (compounds with organic substances);
- changing the composition of the metal by introducing anti-corrosion additives into it);
- modification of the surrounding corrosive environment by introducing inhibitors that affect it.
Electrochemical corrosion protection is the reverse process of electrochemical corrosion. Depending on the shift of the metal potential to the positive or negative side, anodic and cathodic protection are distinguished. By connecting a protector or a direct current source to a metal product, cathodic polarization is created on the metal surface, which prevents the destruction of the metal through the anode.
Electrochemical protection methods consist of two options:
- the metal coating is protected by another metal that has a more negative potential (that is, the protecting metal is less stable than the protected one), and this is called an anodic coating;
- the coating is applied from a less active metal, and then it is called cathode.
Anodic corrosion protection is, for example, galvanized iron. Until all the zinc from the protective layer is used up, the iron will be relatively safe.
Cathodic protection is nickel plating or copper plating. In this case, the destruction of the protective layer also leads to the destruction of the layer that it protects. Attaching a protector to protect a metal product is no different from the reaction in other cases. The protector acts as an anode, and what is under its protectorate remains safe, using the conditions created for it.
Metal coatings
These methods of preventing corrosion involve immersing the steel in a molten metal whose electrical potential is lower than that of iron (the greater the difference, the more effective the coating).
Practical applications include electroplating with zinc or tin, as well as diffusion coatings with nickel, chromium, silicon or aluminum. Compared to other corrosion protection methods, galvanization is known for its lower initial costs, durability and versatility.
Since the consumption of protector metal is quite high, technologies that are characterized by the efficiency of the components used and the strength of the coatings created are advantageous. First on this list is galvanizing. The iron in the steel reacts with the zinc to form a strong alloy coating that serves as protection.
Methods for protecting metal
Electrochemical corrosion is one of the main obstacles encountered in the path of human activity. Protection from the effects of destructive processes and their occurrence on the surfaces of structures and structures is one of the permanent and urgent tasks of any industrial production, and any everyday human activity.
Several methods of such protection have been developed, and all of them are actively used in the daily life cycle:
- Electrochemical protection is an electrolytic operating principle using chemical laws that protects metal using the anodic, cathodic and sacrificial principles.
- Electric spark processing using various installations - non-contact, contact, anodic-mechanical.
- Electric arc spraying is the main advantage in the thickness of the applied layer and the relative cheapness of the process.
- Effective anti-corrosion treatment involves removing contaminants and cleaning the surface being treated, followed by applying first an anti-corrosion layer and then an additional protective layer to the surface.
All these methods have been developed in the process of human activity in order to protect tools, means of transportation and transportation at the junction of several industrial sectors, and using scientific achievements.
Electrochemical corrosion, which is a natural process of destruction of a metal surface under the influence of neutral or aggressive environmental factors, is a complex problem. Machine-building, transport, industrial enterprises and means of transportation suffer losses from it. And this is a problem that requires daily resolution.
More about corrosion and its processes (1 video)
Different types of corrosion (19 photos)
Technical progress in the development of corrosion control methods
Since the loss of metal from corrosion is astronomical, technological progress continues to offer new methods of combating it as research advances and hardware improves . These include:
- thermal spraying, forming ultra-thin protective coatings;
- thermal diffusion coatings that create durable surface protection;
- cadmium plating, which protects steel in sea water.
The growth of industrial production occurs with a constant increase in the production of metal products. Electrochemical corrosion, regardless of the historical era, poses a constant threat to a huge number of structures and critical structures. Therefore, the creation of new methods and means of struggle is one of the tasks of research into technological progress.
Non-metallic coatings
One of the easiest ways to prevent corrosion is to use non-metallic protective coatings such as paint, plastic, wax or powder. Powders, including epoxy resin, nylon and urethane, are applied to a metal surface and heated to the melting stage, forming a thin film.
The paint acts as a coating that protects the metal surface from the electrochemical charge that comes from corrosive compounds. Typically they use a combination of different layers of paint that perform different functions. The primer acts as an inhibitor, the intermediate coat increases the overall thickness of the paint, and the topcoat provides resistance to environmental factors.
Changes in the composition of technical metal and corrosive environment
It consists of special alloying of steel with elements that increase its corrosion resistance. If possible, a lubricant containing anti-corrosion components (reducing agents) is introduced into the mechanical system, which operates in conditions of high temperature and humidity.
An element that has a positive effect on the corrosion resistance of steel is chromium. To realize this effect, steel must contain at least 13% chromium. Every additional 5% chromium provides even better corrosion resistance.
Nickel is the second important element for improving the corrosion resistance of steel, and the addition of nickel also leads to stabilization of austenite. The third important element for increasing corrosion resistance is molybdenum. However, its additives increase the corrosion resistance only of stainless steels with sufficient chromium and nickel content.
Chemical coatings
Refers to methods of temporary anti-corrosion protection of steel, for example, during plastic deformation at elevated temperatures. The most widely used technologies are phosphating and oxalation.
When phosphating, the surface is covered with a continuous layer of phosphate salts of iron and manganese, and when oxalating, it is covered with water-soluble salts of oxalic acid. Phosphating is used for processing unalloyed steels, oxalation – for alloyed ones. The coating adheres firmly to the surface, helping to reduce friction and reduce tool wear. After stamping is completed, the coating is removed.