Electrochemical polishing is the process of treating the surface of a part by immersing it in an acid solution. The metal product is connected to a positively charged anode, and a current of 10–20 V is passed through the electrolyte. As a result, the metal is covered with an oxide or hydroxide film, under which polishing occurs by smoothing out protruding micro-irregularities. Chemical polishing gives approximately the same effect, but here the workpieces are not exposed to the influence of electric current.
The quality of work depends on the homogeneity of the material. Polishing pure metals results in a smooth, shiny product. Polishing complex alloys does not give such a result. At the end of the work, the treated surface increases its roughness purity by 2 classes.
Polishing of parts is carried out only after their visual inspection. It is not allowed to have deep scratches or holes on them, since such defects cannot be eliminated during the polishing process. The best option is to work with cylindrical parts. Flat workpieces are less amenable to polishing.
At the end of the procedure, the products acquire a number of positive qualities: their corrosion resistance increases, the strength of the surface layer increases and the coefficient of friction decreases.
Chemical and electrochemical polishing of metals.
Electrochemical and chemical polishing is used both for decorative surface treatment after coating and during the processing of parts.
Electrochemical polishing.
With electrochemical polishing, the surface microrelief is much smoother than with mechanical processing.
The coatings obtained by electrochemical polishing are non-porous and finely crystalline, which helps reduce the coefficient of friction and makes it possible to impart special optical properties to parts. During electrochemical polishing, the metal surface becomes shiny as a result of different rates of dissolution of microprotrusions and depressions.
The effect of electrochemical polishing is explained by the formation of a thin surface oxide film on the metal, which prevents etching. The thickness of the film is not the same on microprotrusions and microdepressions, as a result of which the solution during electrochemical polishing has a stronger effect on those areas where the film is thinner, i.e. on microprotrusions.
The quality of electrochemical polishing depends on the current density, electrolyte temperature, solution composition and electrolysis time.
The most widely used electrolytes based on phosphoric, sulfuric and chromic acids. To increase the viscosity of solutions, glycerin and methylcellulose are introduced. Sulfureide, triethanolamine, etc. are added to electrolytes for electrochemical polishing as etching inhibitors.
Chemical polishing.
The chemical polishing method has much in common with the electrochemical polishing method. The appearance of shine on the surface of parts here, as with electrochemical polishing, is also associated with the presence of a thin film that prevents etching in the metal recesses.
The preferential dissolution of protrusions during chemical polishing is achieved both due to their increased chemical activity and due to the higher rate of diffusion of metal ions and fresh electrolyte.
Electrochemical polishing of steel parts.
Comparative characteristics of electrochemical and chemical polishing processes.
The main advantages of the electrochemical polishing process are high productivity, good adhesion of galvanic coatings to the electropolished surface, and the ability to eliminate the degreasing operation necessary for mechanical polishing.
The disadvantages of the electrochemical polishing process include the need to frequently change electrolytes due to the lack of a universal one for various metals; the need for mechanical polishing of the surface before electrochemical polishing; increased energy consumption.
The advantage of chemical polishing over electrochemical polishing is that it does not require the use of constant power sources. Chemical polishing is mainly applied to brass or aluminum parts of any complex configuration and size that do not require a mirror finish.
The disadvantages of chemical polishing compared to electrochemical polishing are lower gloss, greater aggressiveness of solutions and their fragility.
Compositions of electrolytes for chemical and electrochemical polishing of metals.
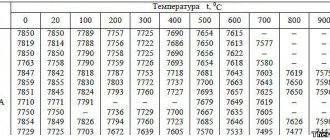
Most electrolytes for electrochemical polishing of steel are based on mixtures of solutions of orthophosphoric and sulfuric acids with the addition of chromic anhydride.
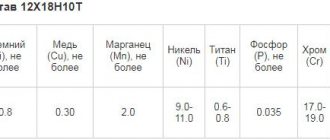
Electrochemical polishing electrolyte containing 500–1100 g/l phosphoric acid, 250–550 g/l sulfuric acid and 30 g/l chromic anhydride is universal for electrochemical polishing of all types of steel, including 12Х18Н9Т. Electrochemical polishing mode: temperature 60–80 0 C, current density 15–80 A/dm 2, time 1–10 minutes.
For electrochemical polishing of steel 12Х18Н9Т it is possible to use electrolytes containing surfactants. Metal removal during electrochemical polishing occurs more intensively in the electrolyte: phosphoric acid 730 g/l, sulfuric acid - 580–725, triethanolamine 4–6 g/l, catapine 0.5–1.0 at 60–80 0 C, current density 20– 50 A/dm2, time 3–5 minutes.
How does the electropolishing process work?
A metal plate is connected to the cathode (-), and the workpiece is connected to the anode (+). The DC power supply creates a low voltage in the acid bath. An electrochemical reaction occurs that causes etching of the metal at the anode. The electric charge is increased on the protrusions - this is how burrs are removed. Electropolishing kills bacteria and improves the corrosion resistance of metal.
The best results are obtained with the correct ratios of voltage density, current density and the use of an effective electrolyte.
Example: electropolished pipes in a dairy plant make it possible to extend the shelf life of the final product because the amount of bacteria and harmful substances in the milk that cause it to sour is reduced.
Aluminum polishing methods: pastes and electropolishing
Polishing aluminum in industrial production conditions. Polishing aluminum using a machine and a special paste. Self-polishing aluminum at home. Chemical and electrochemical processing.
Aluminum polishing is a technical process in which products return to their original appearance. Aluminum is a fairly soft scrap metal, and during use it is often subject to scratches and deformations. To restore the shine and smoothness of the coating to the parts, it is necessary to polish. This article describes not only all industrial methods of polishing aluminum, but also methods for bringing surfaces to a mirror shine at home.
Stainless steel polishing methods
The material contains alloying elements that protect against corrosion and carbon deposits. Over time, scratches and abrasions, as well as oxidation, appear on the surface. Polishing the stainless steel helps with this. In this case, high roughness classes are achieved during processing.
Polishing stainless steel
Methods for polishing stainless steel
Stainless steel polishing can be done at home. In this case, several processing methods are used. Common methods include:
- mechanical;
- electrochemical;
- electrolyte-plasma.
Mechanical restoration
Polishing of stainless steel is carried out using a material represented by grains of abrasive material. When processing, a circle, disk, roller or tape is used.
Various pastes, solutions and suspensions for polishing act as abrasives. The material may contain substances that, in combination with abrasive grains, remove irregularities on metal surfaces.
This type of processing is called mechanical.
As a result of mechanical impacts on the metal surface, grooves and stripes with roughness up to class 7 are formed. In this case, additional refinement of stainless steel to class 10 by grinding is necessary.
Refinement of stainless steel can be done at home without the use of special devices and tools. This type of polishing is common in private workshops and garages. In industrial enterprises, the following types of tools are used:
- manual devices with electric and pneumatic drive;
- polishing and grinding machines;
- drum and vibration units;
- installations for processing using magnetic abrasive.
The following abrasive materials are used for fine grinding:
- liquid polish;
- pasta;
- suspension.
They contain mineral oils, paraffin and stearin additives as a base; they must be removed after processing with solvents.
Electrochemical method
Chemical polishing is the process of removing roughness using the ordered movement of charged particles from one electrode to another.
The method uses installations with baths filled with an electrolyte solution. One of the electrodes is connected to the negative pole of the power source.
The immersed stainless metal workpiece is connected to the positive terminal of the power source.
When direct current is applied, charged ions begin to form on the metal surface, which then flow to the cathode. When stainless steel particles are released, the microprotrusions are smoothed out. During processing, the operator can set the depth of metal removal by adjusting the constant current value, as well as the process time.
The method allows you to polish parts with complex geometric surfaces. Uneven areas are removed from difficult to access areas. The electrolyte has a temperature of up to 90°C, a current density of 0.5 A/cm2, and contains inorganic acids: phosphoric acid and sulfuric acid.
Electrolyte-plasma polishing
The method is based on the formation of a jacket on top of the part, which is a vapor-gas plasma. This allows you to remove irregularities from the metal surface. Apparatuses for polishing stainless steel at home operate on an alternating current network at a voltage of 400 V and an electrolyte solution temperature of 90°C. The rate of metal layer removal is up to 3 microns per minute.
The advantages of this method include:
- use of safe substances;
- minimum costs.
Polishes
Grinding of stainless steel is carried out using hand tools with an electric drive. The following are used as additional accessories:
- a circle of felt or felt, a napkin, and also a disk;
- roller;
- abrasive sheet, disc with an abrasive base;
- non-woven materials;
- polishing tapes.
The tools are:
- orbital sanders;
- grinders with a set of attachments;
- belt type machines;
- tape cutter for direct processing;
- portable sanders;
- tape-type files with the ability to rotate the attachments.
Stainless steel polishing products
Frequency of appearance care
The frequency of polishing stainless steel depends on the occurrence of damage and abrasions on the metal surface. After processing, a protective film of chromium atoms is formed on the stainless steel, which prevents corrosion and rusting. This creates a matte finish.
To preserve the metallic shine on the metal surface, it is prohibited to use pastes containing coarse abrasives or chlorine. Damage to metal is detected visually.
How to polish stainless steel to a mirror at home
Polishing stainless steel in a private workshop to a mirror finish is considered affordable. The processing time depends on the number of scratches on the surface, as well as the presence of metal oxidation. Chemical polishing is not recommended as it may be harmful to humans. To process your product with your own hands until it shines you need:
- Install a polishing wheel with fine abrasive on the grinding machine.
- Choose a polish for stainless steel without wax; it is recommended to use abrasive grains of a minimum size in the composition.
- Pour polish onto the circle.
- Bring the device to the left corner of the product.
- Apply power to the machine by pressing the start button.
- The device must be moved in a circular motion.
- After polishing, turn off the power, and then use a rag to remove the remaining polish by wiping away any roughness.
Compliance with stainless metal processing technology will help to obtain a surface without roughness up to class 14. At the same time, the metal acquires a mirror shine.
Support the channel, just read our articles, and we will post useful information about metals for you! Also visit our website, where you will find a lot of information about metals, alloys and their processing.
Methods for polishing aluminum
It is necessary to polish aluminum due to the physical and ergonomic characteristics of this type of metal. During constant use, products are often susceptible to chips and scratches, loss of glazing, oxidation and loss of original shine. To return the parts to their former appearance, they simply need to be polished.
The following polishing methods are widely known at the industrial level:
- chemical and electrochemical;
- electrochemical polishing;
- decorative etching.
These polishing methods are not suitable for use at home, as they involve the use of chemicals that are dangerous for home use, as well as specialized machines for grinding metal surfaces.
How are aluminum and aluminum alloys processed?
Aluminum is one of the most popular metals from which many different parts are made. It is lightweight, durable, does not corrode, and is also easy to process.
Aluminum processing: types and features
Processing of aluminum blanks is possible in several ways, depending on the tasks and the desired result. Most often used:
- chemical polishing;
- electrochemical grinding;
- chemical oxidation.
Chemical polishing and its features
Chemical polishing can eliminate almost any visible surface defects without increasing its reflectivity. The essence of the procedure is that aluminum parts are immersed in a container with a special composition, under the influence of which the surface contour improves and irregularities become less noticeable. Before loading into an alkaline solution, all parts are thoroughly degreased.
Keep the parts in the solution for one to four minutes. The solution temperature is 100 degrees Celsius. After removal, all parts are thoroughly washed, first in hot and then in cold water.
Electrochemical grinding and its features
Most often, the BRYTAL method is used for electrochemical grinding of aluminum workpieces, the essence of which is that each part is first degreased, then carefully washed, and then immersed in an 80-degree solution containing sodium carbonate (15%) and trisodium phosphate (5%).
It is important to have a double impact here: first, by immersing the workpieces in the working solution for 20-30 seconds, the natural layer of aluminum oxide should be removed. After this, a 24-volt discharge is passed between the cathode and anode (which is the aluminum part), thus creating some kind of polarization.
The anode remains covered with an oxidized layer, which, in turn, is dissolved by the electrolyte over time. This takes him approximately the same amount of time as it takes to create, and the thickness of the layer does not increase.
Each part treated in this way is then dried. The result is a fairly thin oxidized layer. By itself, it is not a strong enough protection, and often requires subsequent anodization. As a result of this process, the surface of the workpieces acquires a reflective surface, which is valued, for example, in the manufacture of parabolic headlights. In addition, such products have a high level of wear protection.
What you need to polish aluminum at home
For polishing you may also need:
- medium or fine abrasive sandpaper;
- brushes with metal bristles;
- varnish;
- alcohol-containing liquid;
- rags.
Varnish is necessary for the final coating of the part. It will increase wear resistance and protect aluminum from further damage.
Which polish to choose
- Pastes do not contain ammonia, their composition is not as aggressive and dangerous as that of acid solutions used in industrial metal polishing.
- Effectively affects scratches and other defects on the surface of aluminum, returning parts to their original appearance and shine.
- After using a special paste, a protective layer is formed on the surface of the parts, which prevents oxidation over a long period.
There are many different brands of polishes. You should choose based on the nature of the work performed, the types of parts and the price category of the paste itself.
Electroplasma polishing equipment.
The set of electroplasma polishing equipment includes:
- steel bathtub with a special protective casing and lifting mechanism; this layer protects from vapors, and the mechanism ensures convenient and safe immersion of metal products;
- a transformer is connected to the bath (the range is selected in accordance with the performance of the installation, the total area of polished surfaces);
- Additionally, a stand with a control element and monitoring sensors .
The installation allows both mechanical and automatic control. It must be equipped with a protective relay that turns off the equipment in case of overheating. Electroplasma polishing of stainless steel is considered the most effective and safe.
The installation itself is connected to an industrial power supply with a voltage of 380 V and a standard frequency of 50 Hz. The transformer has a power of 400 kW , which corresponds to the size of the submersible metal structures. The bath is connected to a pipeline with running water and a compressed air supply system. Must be equipped with a hood. The entire installation takes up 10 m2 in area.
- There is no need to specially pour a foundation under the EPP.
- The device itself has a simple control system.
Types and advantages of brass products
Products made from brass are famous for their durability and wear resistance when cared for with care and properly coated. Coating often involves applying a top protective layer directly to the metal itself. The choice of protective layer depends directly on the conditions of use of the product. If we talk about buildings or plumbing, the coating materials in this case are zinc, aluminum, chromium, nickel, etc. Also, the protective layer can have a decorative function when it comes to products for interiors or luxury items. To do this, manufacturers can plate brass products with silver or gold by sputtering.
Does brass rust? No, it doesn’t. An important advantage of brass (even a classic alloy without impurities and additives) among other metals is that it does not rust, but only darkens, loses its mirror shine, and oxidizes. Therefore, this metal was widely used and is still used to make faucets, basins, bathtubs, buttons, dishes, orders, medals, figurines, candlesticks, frames for large mirrors or paintings, bases for glass tables, various decorations, etc.
Polishing brass. Gtool Group Technology
Once again, the client presented us with a task, and we eagerly began to solve it.
So, you need to polish sheet brass. The sheet can be of different sizes. For the experiment, we were given a piece measuring +- 330x290 mm.
This is what the sheet looked like before processing.
There are many scratches on the surface of unknown depth.
Let's start processing.
The most inexpensive and accessible solution would be to use an angle grinder with adjustable speed and Velcro wheels of different grain sizes.
We chose an angle grinder that has been proven over the years and by our many clients - FINIMASTER from Cibo.
Cibo Finimaster
I personally really like the shape of the machine - it has a “tail” at the back, which is comfortable to hold on to and makes your hands less tired while working.
For Velcro circles, I am used to using a soft support plate - 50099/1 (Velcro M)
Support plate d125 VELCRO M (Soft)
It works perfectly on both flat and curved surfaces and pipes, taking their shape.
We will use Velcro circles with aluminum oxide AO.
Velcro circles Deerfos JSC
These are the simplest and most inexpensive circles, with a soft paper base. Plus, a single layer of uniform abrasive grain allows you to get a very high-quality and uniform finish.
The first circle is the most important. You need to choose the right abrasive grain so that it removes scratches in a couple of movements. At the same time, do not use something too rough so that you don’t have to remove the surface for a long time after it.
Let's start with P320 grain.
Always try to start with a finer grain than you initially think. If the speed doesn't suit you, you can always take a rougher circle. If you are satisfied with the result, you will save a couple of steps and time.
Recommended speed - 3000 rpm
The P320 wheels did an excellent job of removing scratches and leaving a good surface quality.
Sheet after first step P320
Now let's start reducing the roughness. Step by step we will change the grit of the wheels and prepare the surface for polishing.
To ensure a guaranteed result, I prefer not to take large steps or skip any grain. Therefore, the following circles will go further:
This is the result we get after a circle with P2000 grit.
After R2000
By the way, it was thanks to the new Velcro circles with P1000-2000 grit from Deerfos that we were able to achieve this result.
Velcro circles Deerfos P2000
Previously, our assortment only included wheels up to P800.
A large flat surface requires special quality control of processing. It is very important to leave the part as even as possible. Therefore, after a regular angle grinder, I use an orbital grinder, which works with the entire plane of the wheel.
Bosch GEX 125-1 AE Professional
I chose one of the most inexpensive machines from Bosch - Bosch GEX 125-1 AE Professional.
The machine has a speed control of 7000-12,000 rpm. This is a necessary option to solve our problem.
An important point: angle grinders are less productive compared to angle grinders, so when working in circles with Velcro, I will go back two steps to ensure that there is no risk from grinding with a machine.
We repeat the procedure using Velcro circles P1500 and P2000.
This is what we get in the end.
Surface after OSHM R2000
Looks great already. You can start polishing.
Since brass is a soft metal and very high-quality preparation was carried out, I will immediately polish it with a paste for non-ferrous metals and stainless steel 3M Marine 09019.
Polishing paste 3M Marine 09019
The ideal solution for this paste is a 3M soft polishing pad - Finesse-it d125 soft felt polishing pad.
For initial polishing, we again use a Finiteasy angle grinder with a soft support plate.
Polishing with 3M Marine paste (2000 rpm)
During the polishing process, we observe how the risks from the grinding wheels disappear. As necessary, add paste to the circle and repeat the operation.
Here is the result.
After initial polishing.
Brass already looks great, but small marks remain on the surface from the polishing wheel itself and particles of metal or dirt that have gotten into it.
To remove these defects, we will again use the Bosch OSHM and use a paralon polishing wheel for black paintwork. This combination is excellent - the OSH has a trajectory of movement, the traces of which are almost invisible, and nothing can be softer than such a circle. Apply 3M Marine paste again and polish the part for a couple of minutes (at low speeds - 7500 rpm).
Polishing using OSHM.
After OSHM, a little paste remains on the sheet, which clouds the shine. The paste needs to be removed.
As it turned out, simply wiping the brass will not work - it will immediately get scratched.
Then I took the orbital again and stuck a small piece of clean rags to it.
We apply Cibo Viennese lime to the surface (the best solution for removing polishing paste residues!) and again at the lowest speed we finally clean the surface.
Removing polishing paste residues with Viennese lime.
That's it, now the result is perfect.
The final result.
The part has a smooth surface that does not distort the image and there are practically no traces left from mechanical polishing. That's what we wanted.
Here's a video of the process:
The latest news on our social networks and on our channel:
G Group Youtube
https://www.facebook.com/Gtoolgroup/
https://www.instagram.com/g.ru/