Specific volume is the volume per unit weight of a given substance. Dimension: m 3 / kg The reciprocal of the specific volume is the specific gravity. Dimension: kg/m3 In addition to the specific volume, the state of the body can be characterized by molar volume = specific volume x μ, where μ is the molecular weight of the substance.
Methods for experimental determination of specific volumes of substances
Various methods are used: a method based on weighing, a pycnometer method, a hydrometer method and others, depending on the state of aggregation of the substance under study, pressure and temperature, as well as the possible conditions of the experiment.
Determination of metal specific gravity
The body under test is first weighed in the air, let its weight be = g1, and then immersed in water. Due to the loss in weight of the object (according to Archimedes' law), the scale pan to which the body is suspended rises. To bring the scales to the equilibrium position, it is necessary to put some weight - g2. Body specific gravity = g1 : g2
The body under test can have any shape, but it must not be too small (so that, in comparison with its weight, the weight of the thread used for hanging can be neglected).
Example: A piece of high-speed steel weighs g1 = 450 g Additional load g2 = 55 g Specific gravity ϒ = g1/g2 = 450/55 = 8.3 g/cm 3
Cast iron has become quite widespread. Like other metals, it has a fairly large number of physical and mechanical properties, among which specific gravity can be noted. This indicator is often taken from technical literature in the production of a wide variety of products.
Definition and characteristics of density
Density is a physical quantity that determines the ratio of mass to volume. Almost all materials are characterized by a similar physical and mechanical indicator. It is worth considering that the corresponding density of aluminum, copper and cast iron differ significantly. The considered physical and mechanical quality determines:
- Some physical and mechanical properties. In most cases, an increase in density is associated with a decrease in the grain structure. The smaller the distance between individual particles, the stronger the bond formed between them, the hardness increases and the ductility decreases.
- As the distance between particles decreases, their number and weight of the material increase. Therefore, when creating cars, airplanes and other equipment, a material is selected that is lightweight and sufficiently durable. For example, the density of aluminum kg m3 is about 2,700, while the density of metal kg m3 is more than twice that.
There are special tables of metal density , which indicate the indicator in question for steel and non-ferrous alloys, as well as cast iron.
Distribution and use of cast iron
Cast iron came into widespread use many years ago. This is due to the fact that the material is quite simple to produce and has quite attractive performance properties. The following varieties of this material are distinguished:
- High-strength: used in the production of products that must have increased strength. A similar structure is obtained by adding magnesium impurities to the composition. It is highly resistant to bending and other impacts not associated with variable loads.
- Malleable cast iron: has a structure that is easily forged due to its high ductility. The production process involves annealing.
- Half: has a heterogeneous structure , which largely determines the basic mechanical qualities of the material.
The specific gravity largely depends on the production method used, as well as the chemical composition. The properties of cast iron are affected by the following impurities:
- When sulfur is added to the composition, the refractoriness decreases and the fluidity value increases.
- Phosphorus allows the material to be used for the manufacture of various complex products . It is worth considering that by adding phosphorus to the composition, strength is reduced.
- Silicon lowers the melting point and significantly improves casting properties.
- Manganese can increase strength and hardness, but adversely affects casting properties.
When considering cast iron, it is worth paying attention to the following information:
- Gray cast iron grade SCh10 is the lightest of all produced: 6800 kg/m 3 . As the grade increases, the specific gravity also increases.
- The malleable variety of this metal has a value of 7000 kg/m3.
- High strength has a value of 7200 km/m 3.
The density of metals, like other materials, is calculated using a special formula. It has a direct bearing on specific gravity. Therefore, these two indicators are often compared with each other.
Chemical composition
This metal is an alloy of iron and carbon that contains small amounts of impurities. The percentage of iron reaches levels of more than 90%. Silicon, phosphorus, manganese and sulfur are also present. Carbon - no less than 2.14%. It defines the properties of the entire connection.
The role of carbon
First of all, carbon gives hardness. It is carbon that forms the strength characteristics of the alloy, which is an excellent material for foundry production. But it also reduces ductility and malleability.
Therefore, a hard but brittle metal has a limited range of applications. These are mainly metallurgy, mechanical engineering, automotive manufacturing, production of heavy special equipment, utilities and industrial design.
In cast iron, carbon is present in different forms: as cementite (Fe 3 C), or graphite (lamellar, spherical, flake). Graphite largely determines the properties of this material, which is currently divided into the following types:
Features of the table used
In order to calculate the weight of the future product, which will be made from cast iron, you should know its dimensions and density index. Linear dimensions are determined in order to calculate the volume. A calculation method is used to determine the weight of a product in cases where it is not possible to weigh it.
When considering methodological tables, it is worth paying attention to the following points:
- All metals are divided into several groups.
- For each material, the name and GOST are indicated.
- Depending on the melting point, the density value is indicated.
- To determine the physical value of specific gravity in kilograms or other changes, conversion of units of change is carried out. For example, if you need to convert grams to kilograms, then multiply the table value by 1000.
Determination of specific gravity is often done in special laboratories. This value is rarely used when carrying out actual calculations during the manufacture of products or the construction of structures.
Application area
The first use of lead - in the manufacture of water pipes and household items, fortunately, dates back to quite a long time ago. Today, metal enters a home only with a protective layer and in the absence of contact with food, water and humans.
- But the use of lead for alloys and as solder began at the dawn of civilization and continues to this day.
- Lead is a metal of strategic importance, especially since bullets began to be cast from it. Ammunition for small arms and sporting weapons is still made only from lead. And its compounds are used as explosives.
- 75% of the world's metal production is used to produce lead-acid batteries. The substance continues to be one of the main elements of chemical current sources.
- The corrosion resistance of the metal is exploited in the manufacture of acid-resistant equipment, pipelines, and protective sheaths for power cables.
- And, of course, lead is used in the equipment of X-ray rooms: cladding of walls, ceilings, floors, protective partitions, protective suits - everything is made with the participation of lead. At testing sites, including nuclear ones, metal is indispensable.
Read also: Is it possible to sharpen serving knives with serrations?
The cost of metals is determined on several world-wide exchanges. The most famous is the London Metal Exchange. The cost of lead in October 2016 is $2087.25 per ton.
Lead is a metal that is in great demand in modern industry. Some of its qualities—corrosion resistance, the ability to absorb hard radiation—are completely unique and make the metal irreplaceable despite its high toxicity.
This video will tell you what happens if you pour lead into water:
The main characteristic affecting the weight of a metal is its density.
What does metal density mean?
The density of a metal refers to its weight per unit of occupied volume. Volume is often measured in cubic meters and cubic centimeters. What is the reason for such large, by earthly standards, weight and density? The density of a metal and its weight depend on how small the radius of the atom is and how large its weight is.
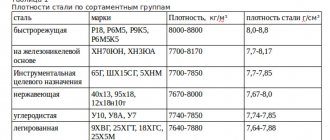
Density of metals table
| Metal | g/cm 3 | kg/m 3 | Metal | g/cm 3 | kg/m 3 |
| Lithium | 0,534 | 534 | Samarium | 7,536 | 7536 |
| Potassium | 0,87 | 870 | Iron | 7,87 | 7874 |
| Sodium | 0,968 | 9680 | Gadolinium | 7,895 | 7895 |
| Rubidium | 1,53 | 1530 | Terbium | 8,272 | 8272 |
| Calcium | 1,54 | 1540 | Dysprosium | 8,536 | 8536 |
| Magnesium | 1,74 | 1740 | Niobium | 8,57 | 8570 |
| Beryllium | 1,845 | 1845 | Cadmium | 8,65 | 8650 |
| Cesium | 1,873 | 1873 | Holmium | 8,803 | 8803 |
| Silicon | 2,33 | 2330 | Nickel | 8,9 | 8900 |
| Bor | 2,34 | 2340 | Cobalt | 8,9 | 8900 |
| Strontium | 2,6 | 2600 | Copper | 8,94 | 8940 |
| Aluminum | 2,7 | 2700 | Erbium | 9,051 | 9051 |
| Scandium | 2,99 | 2990 | Thulium | 9,332 | 9332 |
| Barium | 3,5 | 3500 | Bismuth | 9,8 | 9800 |
| Yttrium | 4,472 | 4472 | Lutetium | 9,842 | 9842 |
| Titanium | 4,54 | 4540 | Molybdenum | 10,22 | 10220 |
| Selenium | 4,79 | 4790 | Silver | 10,49 | 10490 |
| Europium | 5,259 | 5259 | Lead | 11,34 | 11340 |
| Germanium | 5,32 | 5320 | Thorium | 11,66 | 11660 |
| Arsenic | 5,727 | 5727 | Thallium | 11,85 | 11850 |
| Gallium | 5,907 | 5907 | Palladium | 12,02 | 12020 |
| Vanadium | 6,11 | 6110 | Ruthenium | 12,4 | 12400 |
| Lanthanum | 6,174 | 6174 | Rhodium | 12.44 | 12440 |
| Tellurium | 6,25 | 6250 | Hafnium | 13,29 | 13290 |
| Zirconium | 6,45 | 6450 | Mercury | 13,55 | 13550 |
| Cerium | 6,66 | 6660 | Tantalum | 16,6 | 16600 |
| Antimony | 6,68 | 6680 | Uranus | 19,07 | 19070 |
| Praseodymium | 6,782 | 6782 | Tungsten | 19,3 | 19300 |
| Ytterbium | 6,977 | 6977 | Gold | 19,32 | 19320 |
| Neodymium | 7,004 | 7004 | Plutonium | 19,84 | 19840 |
| Zinc | 7,13 | 7130 | Rhenium | 21,02 | 21020 |
| Chromium | 7,19 | 7190 | Platinum | 21,40 | 21400 |
| Tin | 7,3 | 7300 | Iridium | 22,42 | 22420 |
| Indium | 7,31 | 7310 | Osmium | 22,5 | 22500 |
| Manganese | 7,44 | 7440 |
The table shows that the specific gravity of a cube of metal varies greatly. The difference in weight between the heaviest and lightest metal is 42 times. Osmium, whose weight is 22500 kg per m 3 and lithium, which has the lowest density, whose weight is 534 kg per m 3. The metal that has the greatest density also has the greatest weight and it is osmium, as we already understood.
The average density among all metals is 11.5 g per cm3.
It is also noteworthy that there are metals whose density is less than water. There are several of them: lithium, potassium, sodium.
For reference, we can add that osmium is not only the heaviest, but also the rarest. It is mined at around 100 kg per year.
Cast iron material: basic properties and important characteristics
Cast iron consists of carbon, iron and some impurities. It is one of the main materials of ferrous metallurgy. Cast iron is used in the manufacture of household and utility items, machine parts and in other industries. It is used in production, focusing and taking into account its properties and characteristics.
This article is precisely intended to tell you about the density of high-strength, liquid, white and gray cast iron, its melting points and specific heat capacity will also be considered separately.
Thermal properties of cast iron
Cast iron, like any metal, has the following properties: thermal, physical, mechanical, hydrodynamic, electrical, technological, chemical. Let's look at each properties in more detail.
This video talks about the structure and composition of cast iron alloys and the dependence of their properties on a specific composition:
Heat capacity
The thermal capacity of cast iron is determined using the displacement rule. When the heat capacity of cast iron reaches a temperature period, the beginning of which begins at a temperature whose value is greater than phase transformations and ends at a level equal to the melting temperature, then the heat capacity of cast iron takes on a value of 0.18 cal/Ho C.
If the value of the melting temperature exceeds the absolute value, then the heat capacity is equal to 0.23 ± 0.03 cal/Ho C. If the solidification process occurs, then the thermal effect is equal to 55 ± 5 cal. The thermal effect depends on the amount of pearlite when pearlite transformation occurs. Typically it takes a value of 21.5 ± 1.5 cal/G.
The volumetric heat capacity is taken to be the product of the specific gravity and the specific heat capacity. For solid cast iron this value is 1 cal/cm 3 *ºС, for liquid cast iron – 1.5 cal/cm 3 *ºС.
Specific heat capacity of cast iron and other metals in table form
Thermal conductivity
Unlike heat capacity, thermal conductivity is not determined by the displacement rule. Only if the amount of graphitization changes, the composition of the cast iron will affect the thermal conductivity.
Thermal diffusivity
The value of the thermal diffusivity of solid cast iron (for large calculations) can be taken equal to its thermal conductivity, and that of liquid cast iron - 0.03 cm 2 * /sec.
Read below about the melting point of cast iron.
Melting temperature
Cast iron melts at a temperature of 1200ºС. This temperature value is 300 degrees lower than the melting point of steel. With an increased carbon content, this chemical element has a close connection at the molecular level with iron atoms.
During the process of melting cast iron and its crystallization, the carbon component cannot completely penetrate the structural lattice of iron. As a result, the material cast iron takes on the property of brittleness. Cast iron is used for parts that require increased strength. However, cast iron is not used in the manufacture of objects that will be subject to constant dynamic loads.
The table below shows the melting point of cast iron in comparison with other metals.
Melting point of cast iron and other metals
Types of cast iron
Depending on the state of carbon in cast iron, there are:
- The most common is gray cast iron. It has high strength, low shrinkage, low crystallization temperature, and is easy to process. It produces high-quality housings and parts for mechanical engineering (pistons, cylinders, boiler bodies and shut-off valves). Cast iron parts that work with shock-free loads have also proven themselves well: machine tool beds, various shafts and pulleys. Carbon content - from 2.4 to 3.8%. Marking - MF.
- High-strength cast iron (DC) is produced using special heat treatment and the addition of additives (alloying). The graphite in it has a spherical shape and, when melted, combines with the elements of the iron crystal lattice. This improves mechanical properties, which makes it possible to produce reliable crankshafts, cylinder covers, cast pipes and heating devices. According to its characteristics, this type is close to some grades of steel.
- Malleable cast iron is used for the manufacture of artistic products, metal decoration, but mainly for the production of manifolds and the production of parts for agricultural machinery and cars that have to work in difficult conditions. Along with others, it is used in the electrical industry. This alloy is a variety of white.
- White cast iron. So named because of the characteristic white color at the fracture sites. Contains about three percent carbon in the form of carbide and cementite. It is fragile and brittle, therefore it is used in the manufacture of parts that are not subject to special loads.
- The transitional stage between MF (gray) and BC (white) is half cast iron. It contains graphite and carbide in equal proportions, with a total carbon content of 3.5-4.15%. The material is used in the production of parts operating under friction conditions.
Read also: Homemade winches and self-pullers
Mechanical Features
Tensile strength
The compressive strength of cast iron depends on the structure of the material itself. The components of the structure gain their strength along with an increase in the level of dispersion. The tensile strength is strongly influenced by the number, size, distribution and formagraphite inclusions. The tensile strength decreases by a noticeable amount if the graphite inclusions are arranged in the form of a chain. This arrangement reduces the cohesion of the metal mass.
The tensile strength reaches its maximum value when the graphite takes on a spheroidal shape. This form is obtained without the influence of temperature, but when cerium and magnesium are included in the cast iron mass.
- When the melting temperature increases to 400ºС, the tensile strength does not change.
- If the temperature rises above this value, the tensile strength decreases.
- Note that at temperatures from 100 to 200ºС, the tensile strength can decrease by 10-15%.
Plastic
The ductility of cast iron largely depends on the shape of the graphite, and also depends on the structure of the metal mass. If graphite inclusions have a spheroidal shape, then the percentage of elongation can reach 30.
- In ordinary gray cast iron, the elongation reaches only a tenth.
- In annealed gray cast iron, the elongation is 1.5%.
Elasticity depends on the shape of the graphite. If the graphite inclusions did not change, and the temperature increased, then the elasticity remains at the same value.
The elastic modulus is considered a conditional value, since it has a relative value and directly depends on the presence of graphite inclusions. The elastic modulus decreases if the number of graphite inclusions increases. Also, the elastic modulus increases if the shape of the inclusions is distant from the globular shape.
Impact strength
This indicator reflects the dynamic properties of the material. The impact strength of cast iron increases:
- when the shape of graphite inclusions is close to spherical;
- when the ferrite content increases;
- when the graphite content decreases.
Endurance limit
The endurance limit of cast iron becomes greater when the frequency of loading increases and the tensile strength becomes greater.
What are the similarities and differences between steel and cast iron?
It is useful to know what the difference between materials is for housewives, because cast iron products are much more expensive than their steel counterparts. It will be a shame to overpay 3-5 times, and in the end, to get deceived by a persistent market trader.
1) Similarity/difference of basic characteristics
What cast iron and steel have in common is the material category. Both the first and second contain carbon and iron. This is where the common features of the alloys end. Even when adding the same amount of alloying elements, the resulting result in terms of characteristics will not be 100% or even 80% similar.
Now for the differences. To make it easier to understand, we will present the data in table form.
| Characteristic | Steel | Cast iron |
| Proportion of carbon in the alloy | More than 2% | Less than 2% |
| The content of non-metallic impurities - sulfur, phosphorus, magnesium and so on. | Minimum | A large number of |
| Fragility | Average | Strong |
| Hardness level | High | Average |
| Strength | Above cast iron | — |
| Malleability level | Above cast iron | — |
| Easy casting | — | Above steel |
| Thermal conductivity | — | Above steel |
| Hardening | Need | Not necessary |
| Processing methods | More cast iron | — |
| Weight | — | More steel |
Already based on the information presented above, you can draw certain conclusions, even just by holding two samples of materials in your hands. The most important distinguishing feature at the composition level is the proportion of carbon. If the alloy contains 2.5% of this element, the alloy is considered cast iron, not steel.
Methods for determining steel and cast iron at home:
Hydrodynamic properties
Dynamic viscosity
Viscosity becomes less if the amount of manganese in cast iron increases.
A decrease in viscosity was also noticed with a decrease in the content of sulfur impurities and other non-metallic components. The process is affected by the temperature value. Thus, the viscosity becomes less when the ratio of two temperatures is directly proportional (the temperature of the experiment and the start of solidification).
Surface tension
This figure is 900±100 dynes/cm2. The value increases as the amount of carbon decreases and undergoes significant changes in the presence of non-metallic components.
Toxicity
Cookware is often made from cast iron. The fact is that cast iron as a material is non-toxic and tolerates temperature changes well.
Chemical properties
The corrosion resistance of a material depends on the external environment and its structure. If we consider cast iron from the side of decreasing electrode potential, then its components have the following arrangement: graphite-cementite, phosphide eutectic-ferrite.
It should be noted that the potential difference between graphite and ferrite is 0.56 V. If the dispersion increases, the corrosion resistance becomes less. With a strong decrease in dispersion, the opposite effect occurs, and corrosion resistance decreases. Alloying elements also affect the resistance of cast iron.
The influence of impurities on the characteristics of the metal
Industrial cast iron contains impurities. These impurities greatly affect the properties, characteristics and structure of cast iron.
- Thus, manganese inhibits the graphitization process. The release of graphite is stopped, as a result, cast iron acquires the ability to bleach.
- Sulfur degrades casting and mechanical properties.
- Sulfides are mainly formed in gray cast iron.
- Phosphorus improves casting properties, increases wear resistance and increases hardness. However, against this background, cast iron still remains fragile.
- Silicon has the greatest influence on the structure of the material. Depending on the amount of flint, white and ferritic cast iron are obtained.
To obtain certain characteristics, special impurities are often introduced into cast iron during its manufacture. Such materials are called alloy cast iron. Depending on the added element, cast iron can be called aluminum, chromium, or sulfur. Basically, elements are introduced with the aim of obtaining a wear-resistant, heat-resistant, non-magnetic and corrosion-resistant material.
This video will compare the properties of cast iron and steel:
What is steel?
There is almost no tool/equipment in the world that does not contain steel. The material fits so tightly into the life of humanity that it is very problematic to imagine our existence without this alloy.
The first samples of the alloy were discovered in Turkey. The items are more than 3,700 years old, which means that steel items were popular even before our era. Wars of the past could not have happened without the use of steel weapons - cheap and durable. Industrial casting technology was developed in the early 19th century by Gentsman.
1) Structure and advantages of the alloy
| Advantages of steel | Disadvantages of the material |
| The material has a good margin of strength and hardness. | Weak corrosion resistance of pure steel grades without special alloying additives. |
| An abundance of properties due to the variability of filling pure iron with alloying impurities + processing methods. | |
| Elasticity with viscosity in an optimal ratio. Ideal for holding static, dynamic and shock loads. | Increased electromechanical corrosion due to the property of storing electrical energy. |
| Just slice, boil and fold. | |
| Long service life due to high safety margin. | Significant weight of steel structures + high risk of defects due to the multi-stage manufacturing process. |
| Simplicity and low cost of casting. |
China, Japan and India are considered the leaders in steel production. Russia is consistently among the top 5 countries in terms of production volumes, including. Taking into account the geography of leading producers, it is easy to guess that Asia is the leader in steel in the world.
Important: steel does not belong to pure chemical elements - it is a compound based on several.
A wide range of steel alloys with varying levels of strength, corrosion resistance and other unique characteristics is possible due to the material's "flexibility" in connection with other components.
Features of steel alloys:
- the mandatory presence of 2 components - carbon and iron. The first is responsible for viscosity, and the second is strength;
- With all the abundance of steel mining methods, completely pure variations of mining do not exist. Any alloy will have up to 1.2% silicon and up to 0.5% manganese. With minor inclusions, such impurities do not greatly affect the properties of the resulting alloy;
- To change the properties of the material, other metals are artificially introduced into the alloys in technologically specified proportions.
It is important to note that to change the characteristics of the alloy, sometimes it is enough to introduce only 5% of the alloying component. This diversity allows you to scale metal production at the owner’s discretion, based on demand or the level of competition within the local/global market.
2) Steel classification
The table below will tell you more about the distribution of steel alloys.
| Classifier | Components | Description |
| Chemistry | Carbon | The distribution occurs depending on the proportion of carbon content. For low-carbon steel types this is no more than 0.3%, and the peak value is no more than 0.7%. |
| Alloyed | Steels with additions of manganese, chromium, nickel, molybdenum and other elements. Low-alloy steel contains no more than 2.5% impurities, and high-alloy steel contains more than 10%. | |
| Structural composition | Perlite | Pure steel grades with a low content of carbon and alloy impurities. |
| Martensitic | Varieties of material with a large number and volume of additives. | |
| Autenite | High content of impurities - high-alloy steel grades. | |
| Deoxidizer | Calm | Steel without ferrous oxide impurities. Due to the high cost of production, it is used only in strategic units of metal structures. |
| Semi-calm | It hardens without boiling, however, there are small inclusions of gas bubbles. Some of them are removed during metal rolling. | |
| Boiling | Steel contains gases that are reflected in the characteristics of the material - cracks during welding and other defects. | |
| Purpose | Construction | Steel grades that require high-quality resistance to static/dynamic loads. The material must be easy to weld. |
| Instrumental | Steels with a high content of alloying components and carbon. There are stamping and cutting subtypes of tool steels. The material has high heat resistance, hardness + wear resistance. | |
| Structural | The composition contains low manganese content. Used to solve most routine tasks during construction. | |
| Impurities | Privates | The inclusion of sulfur is up to 0.06%, and phosphorus is up to 0.08%. |
| Quality | The inclusion of sulfur is up to 0.04%, and phosphorus is up to 0.033% | |
| High Quality | The inclusion of sulfur is up to 0.026%, and phosphorus is up to 0.023% | |
| Particularly high quality | The inclusion of sulfur is up to 0.014%, and phosphorus is up to 0.023% |
Thanks to adjustments to alloying inclusions + a specific manufacturing method, another 20 to 100 grades of material can be distinguished in each group.
3) Steel production + pricing
The table below will tell you about the methods for making steel.
| Method | The essence | Prevalence (out of 5 ★) |
| Martenovsky | It is based on the smelting of cast iron with ore in a special furnace at a temperature of more than 1,900 degrees Celsius - this allows you to burn off excess carbon. Alloying components are added at the end of the process. Next, the material goes for rental. | ★★★★★ |
| Converter-oxygen | Method with increased efficiency. Impurities are “blown out” of cast iron in a furnace using a mixture of oxygen and air. Annealing occurs faster + quality is higher. | ★★★★ |
| Electric melting | The material is melted at a temperature of more than 2,100 degrees. The process takes place in a closed furnace - this eliminates the access of gases. Due to its high cost, it is rarely used, and only for high-alloy grades. | ★★ |
| Straight | Pig iron is blown at the mining site. The fuel is natural gas - a mixture of oxygen, ammonia and other elements. Temperature range -1,000+ degrees. | ★★★ |
After using one of the methods, the steel making process is not completed. The result of the work should be a material with a high margin of safety, and it will not be possible to achieve such results without additional processing methods.
| Direction | Method | Description |
| Thermal | Annealing | Steel undergoes sharp temperature heating, after which it cools at different speeds. |
| Hardening | Overheating + rapid cooling. | |
| Vacation | Addition of hardening to reduce alloy stress. | |
| Normalization | Similar to annealing, but in air. | |
| Thermomechanical | High temperature | Mechanical action occurs while the material maintains heat. |
| Low temperature | Cold rolled steel. First there is heating, then partial cooling to a limit of 400-500 degrees, and further hardening. | |
| Thermochemical | Cementation | The upper part of the steel surface is saturated with carbon, which makes the crust more wear-resistant. |
| Nitriding | Incorporation of nitrogen into the material to obtain a top protective layer. | |
| Cyanidation | Combined saturation of nitrogen and carbon. |
The price of steel is as varied as the number of grades. For convenience, the exchanges use the term “standard steel” - from $230 per 1 ton of material. If we are talking about stainless steel grades, the cost can rise to $2,000 per 1,000 kg or more.
Definition and characteristics of density
Density is a physical quantity that determines the ratio of mass to volume.
Almost all materials are characterized by a similar physical and mechanical indicator. It is worth considering that the corresponding density of aluminum, copper and cast iron differ significantly. The considered physical and mechanical quality determines:
- Some physical and mechanical properties. In most cases, an increase in density is associated with a decrease in the grain structure. The smaller the distance between individual particles, the stronger the bond formed between them, the hardness increases and the ductility decreases.
- As the distance between particles decreases, their number and weight of the material increase. Therefore, when creating cars, airplanes and other equipment, a material is selected that is lightweight and sufficiently durable. For example, the density of aluminum kg m3 is about 2,700, while the density of metal kg m3 is more than twice that.
There are special tables of metal density , which indicate the indicator in question for steel and non-ferrous alloys, as well as cast iron.
Distribution and use of cast iron
Cast iron came into widespread use many years ago. This is due to the fact that the material is quite simple to produce and has quite attractive performance properties. The following varieties of this material are distinguished:
- High-strength: used in the production of products that must have increased strength. A similar structure is obtained by adding magnesium impurities to the composition. It is highly resistant to bending and other impacts not associated with variable loads.
- Malleable cast iron: has a structure that is easily forged due to its high ductility. The production process involves annealing.
- Half: has a heterogeneous structure , which largely determines the basic mechanical qualities of the material.
Metals similar to gold in specific gravity
Some other metals also have a density similar to gold. In particular, tungsten and uranium. Uranium cannot be passed off as a noble gold metal for the following main reasons:
- high radioactivity;
- inaccessibility.
Counterfeiters have more options when working with tungsten. But this metal differs significantly from gold in color and hardness. Despite this, the counterfeiters found a way out. They cover tungsten ingots with molten gold.
In addition, tungsten is often used in the production of gold-plated jewelry. They are very similar in appearance to real gold jewelry, but their cost and durability set them apart from gold jewelry.
Gold plating is also applied to lead products, the structure of which is much softer. The common belief that specific gravity will distinguish a fake lead from a real gold metal is incorrect. This is a misconception, since gold in its pure form is not used to make jewelry.
You can often find gold jewelry with unusual colors on sale. Often these are ordinary sprayings. If the product is made of alloy, then its price will be much higher. For example, gold comes in blue, pink, black, purple and other shades. They are obtained by including other compounds in the ligature.
Today, unscrupulous jewelers do not hesitate to pass off other metals as precious gold. To avoid purchasing a counterfeit, you should only contact specialized stores that have the appropriate certificates and licenses.
Features of the table used
In order to calculate the weight of the future product, which will be made from cast iron, you should know its dimensions and density index. Linear dimensions are determined in order to calculate the volume. A calculation method is used to determine the weight of a product in cases where it is not possible to weigh it.
When considering methodological tables, it is worth paying attention to the following points:
- All metals are divided into several groups.
- For each material, the name and GOST are indicated.
- Depending on the melting point, the density value is indicated.
- To determine the physical value of specific gravity in kilograms or other changes, conversion of units of change is carried out. For example, if you need to convert grams to kilograms, then multiply the table value by 1000.
Determination of specific gravity is often done in special laboratories. This value is rarely used when carrying out actual calculations during the manufacture of products or the construction of structures.
The physical properties of cast iron (density, thermophysical and electromagnetic properties) depend on the composition and structure, and therefore on the type and grade of cast iron.
Metal production
Lead is quite common, forms several industrially important minerals - galena, cerussite, anglesite, so its production is relatively cheap. The metal is obtained by pyrometallurgical and hydrometallurgical methods. The second method is safer, but is used much less frequently, since it is more expensive, and the resulting metal still needs final processing at high temperatures.
Production using the pyrometallurgical method includes the following stages:
- ore mining;
- crushing and enrichment mainly by flotation method;
- smelting for the purpose of obtaining crude lead - reduction, furnace, alkaline, and so on;
- refining, that is, purifying black lead from impurities and obtaining pure metal.
Despite the same production technology, the equipment can be used in very different ways. This depends on the metal content in the ore, production volumes, product quality requirements, and so on.
Read below about the use and price per 1 kg of lead.
Density of cast iron.
By neglecting the relatively small influence of a number of elements in ordinary cast iron, the density of cast iron can be calculated.
where C, S, P are mass fractions of elements,%; Cr—mass fraction of graphite,%; P—porosity, %; 15 Sv; 2.7S; 14.5 (P-0.1) - the amount of iron carbides, manganese sulfides and phosphide eutectic, respectively.
The given formula gives quite satisfactory agreement with experimental data.
In table 1 shows the density of various groups of cast irons.
The highest density is characterized by white cast irons that do not contain free graphite inclusions, and some alloy cast irons (chrome, nickel, chromium-nickel).