Copper is an element of the fourth period of the eleventh group of the corresponding table of elements. Copper in its simplest form is a plastic material of a transitional type with a pink or golden hue.
Copper is one of the very first materials mastered by man, due to its low melting point and mass availability. This material covers the seven metals mastered in ancient times. Copper is found in the form of nuggets more often than iron, silver or gold. The chemical name for copper is Cuprum, derived from the name of the island of Cyprus.
Basic information about copper
Copper is the most common non-ferrous metal. It received its name in Latin – Cuprum – in honor of the island of Cyprus. It was mined there by the ancient Greeks thousands of years ago. Historians even came up with the Copper Age , which lasted from the 4th to 5th centuries BC. e. At that time, people made from popular metal:
In the table D.I. Mendeleev, it ranks 29th. This element has unique properties - physical, chemical and mechanical. In ancient times, copper could be found in the natural environment in the form of nuggets, sometimes of very large sizes. People heated the rock over an open fire and then cooled it sharply. As a result, it cracked, which made it possible to restore the metal. This simple technology made it possible to begin the development of a popular element.
ORIGIN
Small nugget of copper
Typically, native copper is formed in the oxidation zone of some copper sulfide deposits in association with calcite, native silver, cuprite, malachite, azurite, brochantite and other minerals. The masses of individual clusters of native copper reach 400 tons. Large industrial deposits of native copper, along with other copper-containing minerals, are formed when volcanic rocks (diabases, melaphyres) are exposed to hydrothermal solutions, volcanic vapors and gases enriched in volatile copper compounds (for example, the Lake Superior deposit, USA). Native copper is also found in sedimentary rocks, mainly in cuprous sandstones and shales. The most famous deposits of native copper are the Turin mines (Urals), Dzhezkazgan (Kazakhstan), in the USA (on the Keweenaw Peninsula, in the states of Arizona and Utah).
Properties
Copper is a non-ferrous metal of a reddish color with a pink tint , endowed with high density. There are more than 170 types of minerals in nature that contain Cuprum. Only 17 of them undergo industrial mining of this element. The bulk of this chemical element is contained in ore metals:
- chalcocine - up to 80%;
- bronitite - up to 65%;
- Kovelin - up to 64%.
From these minerals copper is enriched and smelted. High thermal conductivity and electrical conductivity are the distinctive properties of non-ferrous metal. It begins to melt at a temperature of 1063 o C, and boils at 2600 o C. The Cuprum brand will depend on the production method. Metal happens:
Each type has its own special parametric calculations that characterize the degree of shear resistance, deformation under the influence of loads and compression, as well as the tensile elasticity of the material.
Non-ferrous metal actively oxidizes during heating. At a temperature of 385 o C, copper oxide is formed. Its content reduces the thermal conductivity and electrical conductivity of other metals. When interacting with moisture, the metal forms cuprite, and with an acidic environment - vitriol.
Copper Specific Gravity
Due to its properties, this chemical element is actively used in the production of electrical and electronic systems and many other products for other purposes. The most important property is its density of 1 kg per m 3 , since this indicator is used to determine the weight of the product being manufactured. Density shows the ratio of mass to total volume.
The most common system for measuring density units is 1 kilogram per m3. This figure for copper is 8.93 kg/m3. In liquid form, the density will be 8.0 g/cm 3 . The overall density may vary depending on the type of metal that has various impurities. For this purpose, the specific gravity of the substance is used. It is a very important characteristic when it comes to the production of materials that contain copper. Specific gravity characterizes the ratio of the mass of copper in the total volume of the alloy.
Read also: What is the pressure in a beer keg?
The specific gravity of copper will be 8.94 g/cm3 . The specific density and weight parameters of copper are the same, but such a coincidence is not typical for other metals. Specific gravity is very important not only in the production of products containing it, but also in the processing of scrap. There are many techniques that can be used to rationally select materials for forming products. In international SI systems, the specific gravity parameter is expressed in newtons per 1 unit of volume.
It is very important to carry out all calculations at the design stage of devices and mechanisms. Specific gravity and weight are different values, but they are necessarily used to determine the mass of blanks for various parts that contain Cuprum.
If we compare the density of copper and aluminum , we see a big difference. For aluminum, this figure is 2698.72 kg/m 3 at room temperature. However, as the temperature increases, the parameters become different. When aluminum transforms into a liquid state when heated, its density will be in the range of 2.55−2.34 g/cm 3 . The indicator always depends on the content of alloying elements in aluminum alloys.
Technical indicators of metal alloys
The most common copper-based alloys are brass and bronze . Their composition is also formed from other elements:
All alloys differ in structure. The presence of tin in the composition allows the production of bronze alloys of excellent quality. Cheaper alloys include nickel or zinc. The produced materials based on Cuprum have the following characteristics:
- high ductility and wear resistance;
- electrical conductivity;
- resistance to aggressive environments;
- low coefficient of friction.
Copper-based alloys are widely used in industrial production. They are used to produce dishes, jewelry, electrical wires and heating systems. Materials with Cuprum are often used to decorate the façade of houses and make compositions. High stability and ductility are the main qualities for the use of the material.
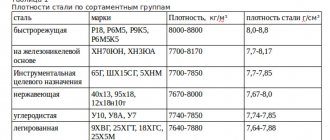
The density of a material is a physical quantity that determines the ratio of the mass of a material to its occupied volume. The unit of measurement for density in the SI system is kg/m3.
The values are averaged and are not reference values; the values of the indicated densities vary depending on the medium and measurement conditions.
One of the most common non-ferrous metals used in industry is copper, its Latin name is Cuprum, after the island of Cyprus, where it was mined by the Greeks many thousands of years ago. This is one of the seven metals that were known in ancient times; jewelry, dishes, money, and tools were made from it. Historians even called the period (from the 4th to the 3rd millennium BC) the Copper Age. D.I. Mendeleev put this metal in 29th place in his table, after hydrogen, since copper does not displace it from an acidic environment. Copper is a non-ferrous metal that has unique physical, mechanical, and chemical properties. The density of copper in kg m³ is one of the most important characteristics; it is used to determine the weight of the future product.
Read also: How to store lithium-ion batteries from a screwdriver
RESERVES AND PRODUCTION
Copper specimen, 13.6 cm. Kinawi Peninsula, Michigan, USA
The average copper content in the earth's crust (clarke) is (4.7-5.5) 10−3% (by mass). In sea and river water the copper content is much lower: 3·10−7% and 10−7% (by weight), respectively. Most copper ore is mined by open pit mining. The copper content in the ore ranges from 0.3 to 1.0%. World reserves in 2000 were, according to experts, 954 million tons, of which 687 million tons were proven reserves; Russia accounted for 3.2% of total and 3.1% of confirmed world reserves. Thus, at the current rate of consumption, copper reserves will last approximately 60 years. Copper is obtained from copper ores and minerals. The main methods for obtaining copper are pyrometallurgy, hydrometallurgy and electrolysis. The pyrometallurgical method involves obtaining copper from sulfide ores, for example, chalcopyrite CuFeS2. The hydrometallurgical method involves dissolving copper minerals in dilute sulfuric acid or ammonia solution; From the resulting solutions, copper is replaced by metallic iron.
How is density determined?
The density of any substance is an indicator of the ratio of mass to total volume. The most common system for measuring density is kilogram per cubic meter. For copper this figure is 8.93 kg/m³. Since there are different grades of metal, which differ depending on the impurities of other substances, the overall density may vary. In this case, it is more appropriate to use another characteristic - specific gravity. In measuring systems, this indicator is expressed in different quantities:
Formula for determining the density of a substance
- SGS system - dyn/cm³;
- SI system - n/m³;
- MKSS system - kg/m³
In this case, the following formula can be used to convert values:
1 n/m³ = 1 dyne/cm³ = 0.102 kg/m³.
Specific gravity is an important indicator in the production of various materials containing copper, especially when it comes to its alloys. This is the ratio of the mass of copper to the total volume of the alloy.
You can consider how this indicator is used in practice using the example of calculating the weight of 25 copper sheets, 2000*1000 mm in size, 5 mm thick. First, let's determine the volume of the sheet - 5 mm * 2000 mm * 1000 mm = 10000000 mm3 or 10,000 cm³.
Specific gravity of copper 8.94 g/cm³
We calculate the weight of copper in one sheet - 10,000 * 8.94 = 89,400 g or 89.40 kg.
The mass of rolled copper in the total amount of material is 89.40 * 25 = 2,235 kg.
This calculation scheme is also used when processing scrap metal.
Basic properties
Smelting copper from ore
Copper, as a metal, is obtained by smelting ore; in nature it is difficult to find pure nuggets; mainly enrichment and extraction is carried out from:
- chalcocite ore, in which the copper content is about 80%, this type is often called copper luster;
- Bronite ore, here the metal content is up to 65%
- covellite ore - up to 64%.
In terms of its physical properties, copper is a red-colored metal; a pink tint may be present in the section; it is classified as a heavy metal because it has a high density.
A distinctive characteristic is electrical conductivity. Due to this, the metal is widely used in the manufacture of cables and electrical wires. In this indicator, copper is second only to silver; in addition, there are a number of other physical characteristics:
- hardness - on the Brindel scale equals 35 kgf/mm²;
- elasticity - 132000 MN/m²;
- linear thermal expansion - 0.00000017 units;
- relative elongation - 60%;
- melting point - 1083 ºС;
- boiling point - 2600 ºС;
- thermal conductivity coefficient - 335 kcal/m*h*deg.
The main properties of copper include the elastic modulus, which is calculated by various methods:
| Copper grade | Shear modulus | Young's modulus | Poisson's ratio |
| Cold drawn copper | 4900 kg/mm² | 13000 kg/mm² | — |
| Rolled copper | 4000 | 11000 kg/mm² | 0,31 — 0,34 |
| Cast copper | — | 8400 | — |
The shear modulus is useful to know in the production of materials for the construction industry - it is a value that characterizes the degree of resistance to shear and deformation under the influence of various loads. The modulus calculated using Young's method shows how the metal will behave under uniaxial tension. The shear modulus characterizes the response of a metal to shear load. Poisson's ratio shows how a material behaves under uniform compression.
Read also: RJ45 connector pinout by color
Development of mines for the extraction of copper and other metals
The chemical properties of copper describe the combination with other substances into alloys and possible reactions to an acidic environment. The most significant characteristic is oxidation. This process actively manifests itself during heating; already at a temperature of 375 ºC, copper oxide begins to form, or scale as it is called, which can affect the conductive functions of the metal and reduce them.
When copper reacts with a solution of an iron salt, it turns into a liquid state. This method is used to remove copper coating on various products.
Long stay in water causes cuprite
When copper is exposed to a humid environment for a long time, cuprite, a greenish coating, forms on its surface. This property of copper is taken into account when using metal to cover roofs. It is noteworthy that cuprite performs a protective function; the metal underneath does not deteriorate at all, even for a hundred years. The only opponents of copper roofs are environmentalists. They explain their position by the fact that when copper cuprite is washed off by rainwater into the soil or water bodies, it pollutes it with its toxins, which especially has a detrimental effect on microorganisms living in rivers and lakes. But to solve this problem, builders use drainpipes made of a special metal, which absorbs copper particles and accumulates them, while the water flows free of toxins.
Copper sulfate is another result of chemical action on metal. This substance is actively used by agronomists to fertilize the soil and stimulate the growth of various crops. However, uncontrolled use of vitriol can also have a detrimental effect on the environment. Toxins penetrate deep into the layers of the earth and accumulate in groundwater.
Note:
205* The empirical radius of the copper atom according to [1] and [3] is 128 pm.
206* Covalent radius of copper according to [1] and [3] is 132±4 pm and 117 pm, respectively.
401* The density of copper according to [3] is 8.92 g/cm3 (at 0 °C and other standard conditions, the state of the substance is a solid).
402* The melting point of copper according to [3] and [4] is 1083.4 °C (1356.55 K, 1982.12 °F) and 1083 °C (1356.15 K, 1981.4 °F), respectively.
403* The boiling point of copper according to [3] and [4] is 2567 °C (2840.15 K, 4652.6 °F) and 2543 °C (2816.15 K, 4609.4 °F), respectively.
407* The specific heat of fusion (enthalpy of fusion ΔHmelt) of copper according to [3] and [4] is 13.01 kJ/mol and 13 kJ/mol, respectively.
408* The specific heat of evaporation (enthalpy of boiling ΔHboiling) of copper according to [3] and [4] is 304.6 kJ/mol and 302 kJ/mol, respectively.
Areas of copper use
Due to its mechanical properties, copper has found wide application in various industries, but most often it can be found as an integral part of electrical wires, in heating and air cooling systems, in the production of computer equipment, and heat exchangers.
Industry uses thousands of tons of copper annually
In construction, this metal is used in the manufacture of various structures; the main advantage here is the low volumetric weight of copper. As noted above, non-ferrous metal has found wide application in roofing work, as well as in the manufacture of pipes. The resulting pipes are lightweight and can be transformed, which is especially important when designing water supply and sewerage systems.
The main part of the production of copper products is wire used as a core for electrical or communication cables. Due to the main characteristic of copper - electrical conductivity, it has a high resistance to current, and also has unique magnetic properties - unlike other metals, its particles do not react to a magnet, which sometimes complicates the process of cleaning it. It is worth noting that almost all production of products is based on the processing of secondary raw materials; ore is used extremely rarely.
Meaning for humans
Copper is inherent in the human body initially:
- Participates in the formation of red blood cells, collagen, elastin.
- Activates the endocrine system, slows down the aging of the body.
- Its deficiency is fraught with a slowdown in protein metabolism. This leads to pathologies in the development of the skeleton and blood composition.
It is found in many foods. Beef liver, oysters, sesame seeds, cocoa powder, black pepper, and buckwheat are rich in copper. And also nuts (hazelnuts, walnuts, cashews, peanuts, almonds).