The high thermal conductivity of copper and its other useful characteristics were one of the reasons for the early development of this metal by humans. To this day, copper and copper alloys are used in almost all areas of our lives.
Copper plates
Copper - briefly about thermal conductivity
Thermal conductivity is the process of transferring the energy of particles (electrons, atoms, molecules) of more heated parts of the body to particles of its less heated parts. This heat exchange leads to temperature equalization. Only energy is transferred along the body; matter does not move. A characteristic of the ability to conduct heat is the thermal conductivity coefficient, numerically equal to the amount of heat that passes through a material with an area of 1 m
2
, 1 m thick, in 1 second at a unit temperature gradient.
The thermal conductivity coefficient of copper at a temperature of 20–100 °C is 394 W/(m * K) - only silver is higher. Rolled steel is inferior to copper in this indicator by almost 9 times, and iron - by 6. Various impurities have different effects on the physical properties of metals. For copper, the rate of heat transfer decreases when substances such as:
- aluminum;
- iron;
- oxygen;
- arsenic;
- antimony;
- sulfur;
- selenium;
- phosphorus.
High thermal conductivity is characterized by the rapid spread of heating energy throughout the entire volume of the object. This ability has provided copper with widespread use in any heat transfer systems. It is used in the manufacture of tubes and radiators of refrigerators, air conditioners, vacuum units, and cars to remove excess heat from the coolant. In heating appliances, similar copper products are used for heating.
Copper's ability to conduct heat decreases when heated. The values of the thermal conductivity coefficient of copper in air depend on the temperature of the latter, which affects heat transfer (cooling). The higher the ambient temperature, the slower the metal cools and the lower its thermal conductivity. Therefore, all heat exchangers use forced airflow by a fan - this increases the efficiency of the devices and at the same time maintains thermal conductivity at an optimal level.
This is interesting: Density and specific gravity of copper - units of measurement, weight calculation
Which conducts heat better: aluminum or copper?
Today, radiators are made from a variety of materials, the most common being steel, stainless steel and aluminum.
Always have doubts about which radiator to choose for installation in your home? Obviously, this depends on personal taste, as well as on the requirements that you have set for yourself regarding the quality of the heating of the room.
Aluminum is by far the most environmentally friendly material and has a huge number of advantages.
Differences between copper and aluminum
The main concerns regarding winding material selection reflect five characteristic differences between copper and aluminum:
Table : Five characteristic differences between copper and aluminum
| Parameter | Aluminum | Copper |
| Expansion coefficient per °C x 10 -6 at 20 °C | 23 | 16,6 |
| Thermal Conductivity BTU/ft/h/FFT 2/°F at 20°C | 126 | 222 |
| Electrical conductivity % at 20°C | 61 | 101 |
| Tensile strength n/mm 2 (soft) | 28-42 | 40 |
How to choose a heating radiator: expert advice
In this article we will not consider cast iron radiators, because... they are losing popularity among buyers.
Let's focus on the most popular models.
The material will tell you in detail about the advantages of aluminum and steel batteries.
Aluminum radiators are lightweight
Aluminum radiators are lighter than traditional steel or cast iron radiators, this fact makes it possible to place such a radiator on any wall in the room.
Aluminum batteries can be hung on the wall, even in situations where the thickness does not allow for deep fastening.
This significantly saves the cost of paying for construction work, since they can be hung very quickly and reliably.
Aluminum is a corrosion resistant material
Aluminum is not subject to corrosion, which makes it an ideal material for the production of radiators that are intended to be installed in areas such as bathrooms and kitchens where there is high humidity.
Aluminum conducts heat well
Aluminum heats up quickly, making it an excellent heat conductor.
Aluminum radiators have a low water content, which means that once turned on, such devices give an intense burst of heat and heat up rooms quite quickly.
By installing aluminum radiators, you can quickly achieve the required temperature in the rooms, as they have the shortest response time.
The main advantage is a significant saving in energy costs during the heating season and, as a wonderful bonus, saving money, since aluminum radiators can be turned off while you are away from the house, and when you return home, turn them on and quickly get a warm home without spending a long time waiting.
Aluminum radiators come in a wide range of designs and colors
There is a common belief that efficient heat cannot be beautiful and original. Fortunately, the days when design must give way to superior performance are over.
Aluminum radiators have a diverse range of designs and offer even the most demanding buyer a decent choice.
You can choose your own finishing color that will perfectly match the style of your home, the shape of the radiator will be one hundred percent in harmony with your home or office atmosphere.
Stainless steel
The use of steel for the production of heat exchangers allows us to obtain durable products, which are mainly used for individual heating systems in houses and cottages.
Due to the ability to control the quality of the coolant and the pressure in the system, steel appliances will be an excellent choice for autonomous heating systems.
Provided that high-quality coolant is supplied and the working fluid pressure is moderate, such devices will last more than 30 years.
Connectivity
Oxides, chlorides, sulfides or base metals that are more conductive on copper than on aluminum. This fact makes cleaning and protecting aluminum connectors more important. Some consider copper and aluminum compounds to be incompatible. Also questionable is the mating of connections between the aluminum of the transformers and the copper connection wire.
Expansion coefficient
When temperature changes, aluminum expands almost a third more than copper. This expansion, along with the ductile nature of aluminum, causes some problems for improperly installed bolted connections.
To avoid loosening of the connection, it must be spring-loaded. By using either cup washers or pressure washers, the necessary elasticity during articulation can be achieved without compressing the aluminum.
When using proper fittings, aluminum connections can be equal in quality to copper ones.
Thermal conductivity
Some argue that since the thermal conductivity of copper is higher than aluminum, this has the effect of reducing the hot spot temperature of the transformer winding.
This is only true when the copper and aluminum winding conductors are of the same size, geometry and design.
Therefore, for any power transformer of a given size, the thermal conductivity characteristics of aluminum can be very similar to copper.
What is thermal conductivity
This term means the ability of various materials to exchange energy , which in this case is represented by heat. In this case, energy transfer passes from the hotter part to the colder part and occurs due to:
- Molecules
- Atoms.
- Electrons and other particles of the metal structure.
The thermal conductivity of stainless steel will differ significantly from that of another metal - for example, the thermal conductivity of copper will be different than that of steel.
To indicate this indicator, a special value is used, called the thermal conductivity coefficient. It is characterized by the amount of heat that can pass through a material in a certain unit of time.
Indicators for steel
Thermal conductivity can vary significantly depending on the chemical composition of the metal. The coefficient of this value will be different for steel and copper. In addition, with an increase or decrease in carbon concentration, the indicator under consideration also changes.
There are other features of thermal conductivity:
- For steel that does not have impurities, the value is 70 W/(m* K).
- Carbon and high-alloy steels have much lower conductivity. Due to an increase in the concentration of impurities, it is significantly reduced.
- The thermal effect itself can also affect the structure of the metal. As a rule, after heating, the structure changes its conductivity value, which is associated with a change in the crystal lattice.
The thermal conductivity coefficient of aluminum is much higher, which is due to the lower density of this material. The thermal conductivity of brass also differs from that of steel.
What is copper and brass
Copper is a non-ferrous metal. Its color is reddish-pink, it is pliable when working, soft and malleable. It has high thermal and electrical conductivity, so copper is often used to produce:
- parts of electrical appliances;
- cables;
- radiators.
Copper is not hardened because it becomes hard even after cold forging. It tends to become covered with patina - a green coating that occurs when the ambient humidity is high.
To increase strength, improve a number of other indicators and reduce the cost of the material, impurities are added and an alloy is obtained.
One such alloy is brass .
In the classic version it contains a third of zinc.
Brass is golden yellow, stronger and harder. It does not oxidize so intensively , and is not so plastic.
Effect of carbon concentration
The carbon concentration in steel affects the amount of heat transfer:
- Low carbon steels have a high conductivity index. That is why they are used in the manufacture of pipes, which are then used to create the heating system pipeline. The coefficient value varies from 54 to 47 W/(m* K).
- The average coefficient for common carbon steels is a value from 50 to 90 W/(m* K). That is why such material is used in the manufacture of parts for various mechanisms.
- For metals that do not contain various impurities, the coefficient is 64 W/(m* K). This value does not change significantly under thermal influence.
Thus, the considered indicator for alloyed alloys may vary depending on the operating temperature.
Thermal diffusivity of metals
The table shows the values of the thermal diffusivity coefficient of pure metals depending on temperature. The thermal diffusivity of metals is indicated in the temperature range from -250 to 1600°C in the dimension m 2 /s.
The following metals are considered: aluminum, cadmium, sodium, silver, potassium, nickel, lead, cobalt, beryllium, lithium, antimony, bismuth, magnesium, zinc, tungsten, tin, antimony, iron, platinum, gold, copper, rhodium, molybdenum, tantalum, iridium.
Based on the thermal diffusivity values in the table, we can distinguish metals with the highest and lowest values of this property. A metal such as bismuth has the lowest thermal diffusivity The thermal diffusivity of pure silver is 158.3 m 2 /s at 100°C. This metal has the highest value of this characteristic.
It should be noted that as the temperature of a metal increases, its thermal diffusivity decreases, with the exception of platinum and cobalt.
Source: Industrial Ovens Reference manual for calculations and design. 2nd edition, expanded and revised, Kazantsev E.I. M., “Metallurgy”, 1975. - 368 p.
Importance in everyday life and production
Why is it important to consider thermal conductivity? A similar value is indicated in various tables for each metal and is taken into account in the following cases:
In the manufacture of various heat exchangers. Heat is one of the important carriers of energy. It is used to provide comfortable living conditions in residential and other premises. When creating heating radiators and boilers, it is important to ensure rapid and complete heat transfer from the coolant to the end consumer.- In the manufacture of outlet elements. You can often encounter a situation where you need to remove heat rather than supply it. An example is the case of heat removal from the cutting edge of a tool or gear teeth. To ensure that the metal does not lose its basic performance qualities, rapid removal of thermal energy is ensured.
- When creating insulating layers. In some cases, the material should not conduct thermal energy transfer. For such operating conditions, a metal is selected that has a low heat conductivity coefficient.
The indicator under consideration is determined when testing under various conditions. As previously noted, the thermal conductivity coefficient may depend on the operating temperature. Therefore, the tables indicate several of its values.
Similarities and differences
Brass alloy consists mostly of copper, so it is natural that they are similar not only visually, but also in some properties. The more copper in the alloy, the more similar their colors will be. This is where the exact coincidences end.
Visually, less than 80% copper are easily distinguished . They are slightly similar to gold, as they have a pronounced yellow tint. The more zinc, the lighter the shade.
Because of this, brass is even used to counterfeit or imitate gold . Copper has a main shade of reddish, which often has a pink tint.
With a strong decrease in temperature, brass does not lose its relatively limited ductility and does not become brittle . Conducts electricity and heat worse.
They differ in such a way as hardness .
Copper is softer and more ductile , while brass, on the contrary, is hard and it is difficult to give it any shape without annealing.
The shavings are also different: for brass they are needle- shaped , for copper they are twisted into a spiral .
Let's look at the properties that brass and copper have and whether they have any differences:
| Copper | Brass |
| Plastic, soft | Solid |
| Reddish-brown-pink tint | Golden tone |
| Lower sound on impact | Alt |
| Heavy | Easier |
| The shavings are twisted into a spiral | Needle shavings |
Thermal conductivity of steel, copper, aluminum, nickel and their alloys
Ordinary iron and non-ferrous metals have different structures of molecules and atoms. This allows them to differ from each other not only in mechanical properties, but also in thermal conductivity properties, which, in turn, affects the use of certain metals in various sectors of the economy.
table 2
Steel has a thermal conductivity coefficient at an ambient temperature of 0 degrees. (C) equal to 63, and when the degree increases to 600, it decreases to 21 W/m*degree. Aluminum, under the same conditions, on the contrary, will increase the value from 202 to 422 W/m*deg. Aluminum alloys will also increase thermal conductivity as the temperature increases. Only the value of the coefficient will be an order of magnitude lower, depending on the amount of impurities, and range from 100 to 180 units.
Copper, with a temperature change within the same limits, will reduce thermal conductivity from 393 to 354 W/m*deg. At the same time, copper-containing brass alloys will have the same properties as aluminum ones, and the thermal conductivity value will vary from 100 to 200 units, depending on the amount of zinc and other impurities in the brass alloy.
The thermal conductivity coefficient of pure nickel is considered low; it will change its value from 67 to 57 W/m*deg. Alloys containing nickel will also have a coefficient with a reduced value, which, due to the content of iron and zinc, ranges from 20 to 50 W/m*deg. And the presence of chromium will reduce the thermal conductivity in metals to 12 units, with a slight increase in this value when heated.
The concept of thermal resistance and thermal conductivity coefficient
If thermal conductivity characterizes the ability of metals to transfer the temperature of bodies from one surface to another, then thermal resistance shows an inverse relationship, i.e. the ability of metals to prevent such transfer, in other words, to resist. Air has high thermal resistance. It is he who, most of all, prevents the transfer of heat between bodies.
The quantitative characteristic of the change in temperature of a unit area per unit of time by one degree (K) is called the thermal conductivity coefficient. The international system of units usually measures this parameter in W/m*deg. This characteristic is very important when choosing metal products that must transfer heat from one body to another.
| Metal | Thermal conductivity coefficient of metals at temperature, °C | ||||
| — 100 | 100 | 300 | 700 | ||
| Aluminum | 2,45 | 2,38 | 2,30 | 2,26 | 0,9 |
| Beryllium | 4,1 | 2,3 | 1,7 | 1,25 | 0,9 |
| Vanadium | — | — | 0,31 | 0,34 | — |
| Bismuth | 0,11 | 0,08 | 0,07 | 0,11 | 0,15 |
| Tungsten | 2,05 | 1,90 | 1,65 | 1,45 | 1,2 |
| Hafnium | — | — | 0,22 | 0,21 | — |
| Iron | 0,94 | 0,76 | 0,69 | 0,55 | 0,34 |
| Gold | 3,3 | 3,1 | 3,1 | — | — |
| Indium | — | 0,25 | — | — | — |
| Iridium | 1,51 | 1,48 | 1,43 | — | — |
| Cadmium | 0,96 | 0,92 | 0,90 | 0,95 | 0,44 (400°) |
| Potassium | — | 0,99 | — | 0,42 | 0,34 |
| Calcium | — | 0,98 | — | — | — |
| Cobalt | — | 0,69 | — | — | — |
| Lithium | — | 0,71 | 0,73 | — | — |
| Magnesium | 1,6 | 1,5 | 1,5 | 1,45 | — |
| Copper | 4,05 | 3,85 | 3,82 | 3,76 | 3,50 |
| Molybdenum | 1,4 | 1,43 | — | — | 1,04 (1000°) |
| Sodium | 1,35 | 1,35 | 0,85 | 0,76 | 0,60 |
| Nickel | 0,97 | 0,91 | 0,83 | 0,64 | 0,66 |
| Niobium | 0,49 | 0,49 | 0,51 | 0,56 | — |
| Tin | 0,74 | 0,64 | 0,60 | 0,33 | — |
| Palladium | 0,69 | 0,67 | 0,74 | — | — |
| Platinum | 0,68 | 0,69 | 0,72 | 0,76 | 0,84 |
| Rhenium | — | 0,71 | — | — | — |
| Rhodium | 1,54 | 1,52 | 1,47 | — | — |
| Mercury | 0,33 | 0,09 | 0.1 | 0,115 | — |
| Lead | 0,37 | 0,35 | 0,335 | 0,315 | 0,19 |
| Silver | 4,22 | 4,18 | 4,17 | 3,62 | — |
| Antimony | 0,23 | 0,18 | 0,17 | 0,17 | 0,21 |
| Thallium | 0,41 | 0,43 | 0,49 | 0,25 (400 0) | |
| Tantalum | 0,54 | 0,54 | — | — | — |
| Titanium | — | — | 0,16 | 0,15 | — |
| Thorium | — | 0,41 | 0,39 | 0,40 | 0,45 |
| Uranus | — | 0,24 | 0,26 | 0,31 | 0,40 |
| Chromium | — | 0,86 | 0,85 | 0,80 | 0,63 |
| Zinc | 1,14 | 1,13 | 1,09 | 1,00 | 0,56 |
| Zirconium | — | 0,21 | 0,20 | 0,19 | — |
Application
The state of aggregation of materials has a distinctive structure of molecules and atoms. This is what has a great influence on metal products and their properties, depending on their purpose.
The different chemical composition of components and parts made of iron allows them to have different thermal conductivities. This is due to the structure of metals such as cast iron, steel, copper and aluminum. The porosity of cast iron products promotes slow heating, and the density of the copper structure, on the contrary, accelerates the heat transfer process. These properties are used for rapid heat removal or gradual heating of inert products. An example of using the properties of metal products is:
- kitchen utensils with various properties;
- pipe soldering equipment;
- irons;
- rolling and sliding bearings;
- plumbing equipment for heating water;
- heating devices.
Copper tubes are widely used in radiators of automobile cooling systems and air conditioners used in everyday life. Cast iron radiators retain heat in the apartment, even with an inconsistent supply of coolant at the required temperature. And radiators made of aluminum contribute to the rapid transfer of heat to the heated room.
When high temperatures occur as a result of friction of metal surfaces, it is also important to take into account the thermal conductivity of the product. In any gearbox or other mechanical equipment, the ability to remove heat will allow the mechanism parts to maintain strength and not be subject to destruction during operation. Knowledge of the heat transfer properties of various materials will allow you to competently use certain alloys of non-ferrous or ferrous metals.
More articles on the topic:
Copper connections
Copper (I) oxide Cu2O3 and cuprous oxide (I) Cu2O , like other copper (I) compounds, are less stable than copper (II) compounds. Copper (I) oxide, or cuprous oxide Cu2O, occurs naturally as the mineral cuprite. In addition, it can be obtained as a precipitate of red copper(I) oxide by heating a solution of a copper(II) salt and an alkali in the presence of a strong reducing agent.
Copper(II) oxide, or copper oxide, CuO is a black substance found in nature (for example, as the mineral tenerite). It is obtained by calcination of copper (II) hydroxycarbonate (CuOH)2CO3 or copper (II) nitrate Cu(NO2)2. Copper(II) oxide is a good oxidizing agent.
Copper (II) hydroxide Cu(OH)2 precipitates from solutions of copper (II) salts under the action of alkalis in the form of a blue gelatinous mass. Even with low heating, even under water, it decomposes, turning into black copper (II) oxide. Copper(II) hydroxide is a very weak base. Therefore, solutions of copper (II) salts in most cases have an acidic reaction, and with weak acids copper forms basic salts.
Copper (II) sulfate CuSO4 in the anhydrous state is a white powder that turns blue when it absorbs water. Therefore, it is used to detect traces of moisture in organic liquids. An aqueous solution of copper sulfate has a characteristic blue-blue color. This color is characteristic of hydrated [Cu(H2O)4]2+ ions, therefore all dilute solutions of copper (II) salts have the same color, unless they contain any colored anions. From aqueous solutions, copper sulfate crystallizes with five molecules of water, forming transparent blue crystals of copper sulfate. Copper sulfate is used for electrolytic coating of metals with copper, for the preparation of mineral paints, and also as a starting material in the preparation of other copper compounds. In agriculture, a diluted solution of copper sulfate is used to spray plants and treat grain before sowing to destroy spores of harmful fungi.
Copper (II) chloride CuCl2. 2H2O. Forms dark green crystals, easily soluble in water. Very concentrated solutions of copper (II) chloride are green, diluted solutions are blue-blue.
Copper (II) nitrate Cu(NO3)2.3H2O. It is obtained by dissolving copper in nitric acid. When heated, blue copper nitrate crystals first lose water and then easily decompose, releasing oxygen and brown nitrogen dioxide, turning into copper (II) oxide.
Copper (II) hydroxycarbonate (CuOH)2CO3. It occurs naturally in the form of the mineral malachite, which has a beautiful emerald green color. It is artificially prepared by the action of Na2CO3 on solutions of copper (II) salts. 2CuSO4 + 2Na2CO3 + H2O = (CuOH)2CO3v + 2Na2SO4 + CO2^ Used to obtain copper (II) chloride, for the preparation of blue and green mineral paints, as well as in pyrotechnics.
Copper (II) acetate Cu (CH3COO)2.H2O. It is obtained by treating copper metal or copper(II) oxide with acetic acid. Usually it is a mixture of basic salts of various compositions and colors (green and blue-green). Under the name verdigris, it is used to prepare oil paint.
Complex copper compounds are formed as a result of the combination of doubly charged copper ions with ammonia molecules. A variety of mineral paints are obtained from copper salts. All copper salts are poisonous. Therefore, to avoid the formation of copper salts, copper utensils are coated on the inside with a layer of tin (tinned).
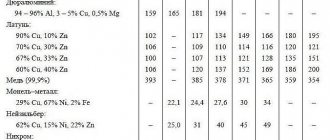
Thermal conductivity of brass and bronze
The table shows the thermal conductivity values of brass, bronze, as well as copper-nickel alloys (constantan, copel, manganin, etc.) depending on temperature - in the range from 4 to 1273 K.
The thermal conductivity of brass, bronze and other copper-based alloys increases when heated. According to the table, L96 brass has the highest thermal conductivity of the alloys considered at room temperature . Its thermal conductivity at a temperature of 300 K (27°C) is 244 W/(m deg).
Also copper alloys with high thermal conductivity include: brass LS59-1, tombac L96 and L90, tin tombac LTO90-1, rolled tombac RT-90. In addition, the thermal conductivity of brass is generally higher than that of bronze. It should be noted that bronzes with high thermal conductivity include: phosphorus, chromium and beryllium bronzes, as well as BrA5 bronze.
The copper alloy with the lowest thermal conductivity is manganese bronze - its thermal conductivity coefficient at a temperature of 27°C is 9.6 W/(m deg).
The thermal conductivity of copper alloys is always lower than the thermal conductivity of pure copper, all other things being equal.
In addition, the thermal conductivity of copper-nickel alloys is particularly low. The most thermally conductive of them at room temperature is cupronickel MNZhMts 30-0.8-1 with a thermal conductivity of 30 W/(m deg). Thermal conductivity table for brass, bronze and copper-nickel alloys
| Alloy | Temperature, K | Thermal conductivity, W/(m deg) |
| Copper-nickel alloys | ||
| Beryllium copper | 300 | 111 |
| Constantan of foreign production | 4…10…20…40…80…300 | 0,8…3,5…8,8…13…18…23 |
| Constantan MNMts40-1.5 | 273…473…573…673 | 21…26…31…37 |
| Kopel MNMts43-0.5 | 473…1273 | 25…58 |
| Manganin of foreign production | 4…10…40…80…150…300 | 0,5…2…7…13…16…22 |
| Manganin MNMts 3-12 | 273…573 | 22…36 |
| Cupronickel MNZHMts 30-0.8-1 | 300 | 30 |
| Nickel silver | 300…400…500…600…700 | 23…31…39…45…49 |
| Brass | ||
| Automatic brass UNS C36000 | 300 | 115 |
| L62 | 300…600…900 | 110…160…200 |
| L68 deformed brass | 80…150…300…900 | 71…84…110…120 |
| L80 semi-tompak | 300…600…900 | 110…120…140 |
| L90 | 273…373…473…573…673…773…873 | 114…126…142…157…175…188…203 |
| L96 tombak drawn | 300…400…500…600…700…800 | 244…245…246…250…255…260 |
| LAN59-3-2 aluminum-nickel brass | 300…600…900 | 84…120…150 |
| LMC58-2 manganese brass | 300…600…900 | 70…100…120 |
| LO62-1 tin | 300 | 99 |
| LO70-1 tin | 300…600 | 92…140 |
| LS59-1 annealed brass | 4…10…20…40…80…300 | 3,4…10…19…34…54…120 |
| LS59-1V leaded brass | 300…600…900 | 110…140…180 |
| LTO90-1 tombak tin | 300…400…500…600…700…800…900 | 124…141…157…174…194…209…222 |
| Bronze | ||
| BrA5 | 300…400…500…600…700…800…900 | 105…114…124…133…141…148…153 |
| BrA7 | 300…400…500…600…700…800…900 | 97…105…114…122…129…135…141 |
| BrAZhMC10-3-1.5 | 300…600…800 | 59…77…84 |
| BrAZHN10-4-4 | 300…400…500 | 75…87…97 |
| BrAZHN11-6-6 | 300…400…500…600…700…800 | 64…71…77…82…87…94 |
| BrB2, annealed at 573K | 4…10…20…40…80 | 2,3…5…11…21…37 |
| BrKd | 293 | 340 |
| BrKMTs3-1 | 300…400…500…600…700 | 42…50…55…54…54 |
| BrMC-5 | 300…400…500…600…700 | 94…103…112…122…127 |
| BrMTsS8-20 | 300…400…500…600…700…800…900 | 32…37…43…46…49…51…53 |
| BrO10 | 300…400…500 | 48…52…56 |
| BrOS10-10 | 300…400…600…800 | 45…51…61…67 |
| BrOS5-25 | 300…400…500…600…700…800…900 | 58…64…71…77…80…83…85 |
| BrOF10-1 | 300…400…500…600…700…800…900 | 34…38…43…46…49…51…52 |
| BrOTs10-2 | 300…400…500…600…700…800…900 | 55…56…63…68…72…75…77 |
| BrOTs4-3 | 300…400…500…600…700…800…900 | 84…93…101…108…114…120…124 |
| BrOTs6-6-3 | 300…400…500…600…700…800…900 | 64…71…77…82…87…91…93 |
| BrOTs8-4 | 300…400…500…600…700…800…900 | 68…77…83…88…93…96…100 |
| Aluminum bronze | 300 | 56 |
| Aged beryllium bronze | 20…80…150…300 | 18…65…110…170 |
| Manganese bronze | 300 | 9,6 |
| Production leaded bronze | 300 | 26 |
| Phosphor bronze 10% | 300 | 50 |
| Phosphor bronze annealed | 20…80…150…300 | 6…20…77…190 |
| Chromium bronze UNS C18200 | 300 | 171 |
Note: Temperatures in the table are given in degrees Kelvin !
Properties of copper Cu: thermal conductivity and density of copper
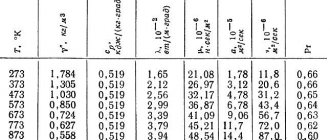
The table shows the thermophysical properties of copper depending on temperature in the range from 50 to 1600 degrees Kelvin.
The density of copper is 8933 kg/m3 (or 8.93 g/cm3) at room temperature . Copper is almost four times heavier than aluminum and iron. These metals will float on the surface of the liquid copper. The copper density values in the table are indicated in kg/m3.
The dependence of copper density on its temperature is presented in the table. It should be noted that the density of copper decreases when it is heated, both as a solid metal and as a liquid copper. The decrease in the density of this metal is due to its expansion when heated - the volume of copper increases. It should be noted that liquid copper has a density of about 8000 kg/m3 at temperatures up to 1300°C.
The thermal conductivity of copper is 401 W/(m deg) at room temperature, which is a fairly high value among metals, comparable to the thermal conductivity of silver.
At 1357K (1084°C) copper goes into a liquid state, which is reflected in the table by a sharp drop in the value of the thermal conductivity coefficient of copper. It can be seen that the thermal conductivity of liquid copper is almost two times lower than that of solid metal.
The thermal conductivity of copper tends to decrease when it is heated, but at temperatures above 1400 K, the thermal conductivity value begins to increase again.
The table discusses the following thermophysical properties of copper at various temperatures:
- copper density, kg/m3;
- specific heat capacity, J/(kg deg);
- thermal diffusivity, m2/s;
- thermal conductivity of copper, W/(m K);
- electrical resistivity, Ohm m;
- Lorentz function;
- heat capacity ratio.
What does thermal conductivity depend on?
Thermal conductivity is a physical quantity and largely depends on the parameters of temperature, pressure and type of substance. Most of the coefficients are determined empirically. Many methods have been developed for this. The results are compiled into reference tables, which are then used in various scientific and engineering calculations. Bodies have different temperatures and during heat exchange it (temperature) will be distributed unevenly. In other words, you need to know how the thermal conductivity coefficient depends on temperature.
Numerous experiments show that for many materials the relationship between the coefficient and the thermal conductivity itself is linear.
Coefficient of thermal conductivity
The thermal conductivity of metals is determined by the shape of its crystal lattice.
In many ways, the thermal conductivity coefficient depends on the structure of the material, the size of its pores and humidity.
When is the thermal conductivity coefficient taken into account?
Thermal conductivity parameters must be taken into account when choosing materials for enclosing structures - walls, ceilings, etc. In rooms where the walls are made of materials with high thermal conductivity, it will be quite cool in the cold season. Decorating the room won't help either. In order to avoid this, the walls must be made quite thick. This will certainly lead to increased costs for materials and labor.
Insulation scheme for a wooden house
That is why the construction of the walls requires the use of materials with low thermal conductivity (mineral wool, polystyrene foam, etc.).
Indicators for steel
- In reference materials on the thermal conductivity of various materials, a special place is occupied by the data presented on steels of different grades. Thus, the reference materials contain experimental and calculated data for the following types of steel alloys: resistant to corrosion and elevated temperatures;
- intended for the production of springs and cutting tools;
- saturated with alloying additives.
The tables summarize the indicators that were collected for steels in the temperature range from -263 to 1200 degrees. The average indicators are for:
- carbon steels 50 – 90 W/(m×deg);
- corrosion-resistant, heat- and heat-resistant alloys classified as martensitic - from 30 to 45 W/(m×deg);
- alloys classified as austenitic from 12 to 22 W/(m×deg).
These reference materials contain information about the properties of cast iron.
Thermal conductivity coefficients of aluminum, copper and nickel alloys
When carrying out calculations related to non-ferrous metals and alloys, designers use reference materials located in special tables.
Table of thermal conductivity of aluminum alloys
They present materials on the thermal conductivity of non-ferrous metals and alloys; in addition to these data, information is provided on the chemical composition of the alloys. The studies were carried out at temperatures from 0 to 600 °C.
According to the information collected in these tabular materials, it is clear that non-ferrous metals with high thermal conductivity include alloys based on magnesium and nickel. Metals with low thermal conductivity include nichrome, invar and some others.
Most metals have good thermal conductivity, some have more, others less. Metals with good thermal conductivity include gold, copper and some others. Materials with low thermal conductivity include tin, aluminum, etc.
Table of thermal conductivity of nickel alloys
High thermal conductivity can be both an advantage and a disadvantage. It all depends on the scope of application. For example, high thermal conductivity is good for kitchenware. Materials with low thermal conductivity are used to create permanent connections of metal parts. There are entire families of tin-based alloys.
Impurities in copper alloys
from here
Impurities contained in copper (and, naturally, interacting with it) are divided into three groups.
Forming solid solutions with copper
Such impurities include aluminum, antimony, nickel, iron, tin, zinc, etc. These additives significantly reduce electrical and thermal conductivity . The grades that are primarily used for the production of conductive elements include M0 and M1. If the copper alloy contains antimony, its hot pressure treatment becomes significantly more difficult.
Impurities that do not dissolve in copper
These include lead, bismuth, etc. Although they do not affect the electrical conductivity of the base metal, such impurities make it difficult to process by pressure.
Melting point of brass
The melting point of brass of the considered brands varies in the range from 865 to 1055 °C. The most fusible is manganese brass LMts58-2 with a melting point of 865°C. Low-melting brasses also include: L59, L62, LAN59-3-2, LKS65-1.5-3 and others.
L96 brass has the highest melting point (1055°C).
Among the refractory brasses, according to the table, we can also distinguish: brass L90, LA85-0.5, tin tombak LTO90-1. Melting point of brass
| Brass | t, °С | Brass | t, °С |
| L59 | 885 | LMts55-3-1 | 930 |
| L62 | 898 | LMts58-2 manganese brass | 865 |
| L63 | 900 | LMtsA57-3-1 | 920 |
| L66 | 905 | LMtsZh52-4-1 | 940 |
| L68 deformed brass | 909 | LMtsOS58-2-2-2 | 900 |
| L70 | 915 | LMtsS58-2-2 | 900 |
| L75 | 980 | LN56-3 | 890 |
| L80 semi-tompak | 965 | LN65-5 | 960 |
| L85 | 990 | LO59-1 | 885 |
| L90 | 1025 | LO60-1 | 885 |
| L96 tombak drawn | 1055 | LO62-1 tin | 885 |
| LA67-2.5 | 995 | LO65-1-2 | 920 |
| LA77-2 | 930 | LO70-1 tin | 890 |
| LA85-0.5 | 1020 | LO74-3 | 885 |
| LAZ60-1-1 | 904 | LO90-1 | 995 |
| LAZHMts66-6-3-2 | 899 | LS59-1 | 900 |
| LAN59-3-2 aluminum-nickel brass | 892 | LS59-1V leaded brass | 900 |
| LANKMts75-2-2.5-0.5-0.5 | 940 | LS60-1 | 900 |
| LZhMts59-1-1 | 885 | LS63-3 | 885 |
| LK80-3 | 900 | LS64-2 | 910 |
| LKS65-1.5-3 | 870 | LS74-3 | 965 |
| LKS80-3-3 | 900 | LTO90-1 tombak tin | 1015 |
Melting point of bronze
The melting point of bronze ranges from 854 to 1135°C. Bronze AZHN11-6-6 has the highest melting point - it melts at a temperature of 1408 K (1135°C). The melting point of this bronze is even higher than the melting point of copper, which is 1084.6°C.
Bronzes with a low melting point include: BrOTs8-4, BrB2, BrMTsS8-20, BrSN60-2.5 and the like.
Melting point of bronze
| Bronze | t, °С | Bronze | t, °С |
| BrA5 | 1056 | BrOS8-12 | 940 |
| BrA7 | 1040 | BrOSN10-2-3 | 1000 |
| BrA10 | 1040 | BrOF10-1 | 934 |
| BrAZH9-4 | 1040 | BrOF4-0.25 | 1060 |
| BrAZhMC10-3-1.5 | 1045 | BrOTs10-2 | 1015 |
| BrAZHN10-4-4 | 1084 | BrOTs4-3 | 1045 |
| BrAZHN11-6-6 | 1135 | BrOTs6-6-3 | 967 |
| BrAZhS7-1.5-1.5 | 1020 | BrOTs8-4 | 854 |
| BrAMTS9-2 | 1060 | BrOTsS3.5-6-5 | 980 |
| BrB2 | 864 | BrOTsS4-4-17 | 920 |
| BrB2.5 | 930 | BrOTsS4-4-2.5 | 887 |
| BrKMTs3-1 | 970 | BrOTsS5-5-5 | 955 |
| BrKN1-3 | 1050 | BrOTsS8-4-3 | 1015 |
| BrKS3-4 | 1020 | BrOTsS3-12-5 | 1000 |
| BrKTs4-4 | 1000 | BrOTsSN3-7-5-1 | 990 |
| BrMG0.3 | 1076 | BrS30 | 975 |
| BrMC5 | 1007 | BrSN60-2.5 | 885 |
| BrMTsS8-20 | 885 | BrSUN7-2 | 950 |
| BrO10 | 1020 | BrХ0.5 | 1073 |
| BrOS10-10 | 925 | BrTsr0.4 | 965 |
| BrOS10-5 | 980 | Cadmium | 1040 |
| BrOS12-7 | 930 | Silver | 1082 |
| BrOS5-25 | 899 | HOT alloy | 1075 |
Note: The melting and boiling points of other common metals are given in this table.
- Physical quantities. Directory. Ed. I.S. Grigorieva, E.Z. Meilikhova. - M.: Energoatomizdat, 1991. - 1232 p.
- Chirkin V.S. Thermophysical properties of nuclear technology materials. M.: Atomizdat, 1967 - 474 p.
Methods for studying thermal conductivity parameters
When studying thermal conductivity parameters, one must remember that the characteristics of a particular metal or its alloys depend on the method of its production. For example, the parameters of a metal produced by casting may differ significantly from the characteristics of a material manufactured using powder metallurgy methods. The properties of raw metal are fundamentally different from those that have undergone heat treatment.
Thermal instability, that is, the transformation of individual properties of the metal after exposure to high temperatures, is common to almost all materials. As an example, metals after prolonged exposure to different temperatures are able to achieve different levels of recrystallization, and this is reflected in the parameters of thermal conductivity.
Steel structure after heat treatment
We can say the following: when conducting studies of thermal conductivity parameters, it is necessary to use samples of metals and their alloys in a standard and specific technological state, for example, after heat treatment.
For example, there are requirements for grinding metal to conduct research using thermal analysis methods. Indeed, such a requirement exists in a number of studies. There are also such requirements - such as the production of special plates and many others.
The non-thermal stability of metals poses a number of limitations on the use of thermophysical research methods. The fact is that this method of conducting research requires heating the samples at least twice, in a certain temperature range.
One of the methods is called relaxation-dynamic. It is designed to perform mass measurements of heat capacity of metals. In this method, the transition curve of the sample temperature between its two stationary states is recorded. This process is a consequence of a jump in thermal power introduced into the test sample.
This method can be called relative. It uses test and comparison samples. The main thing is that the samples have the same emitting surface. When conducting research, the temperature affecting the samples must change in steps, and upon reaching the specified parameters, it is necessary to maintain a certain amount of time. The direction of temperature change and its step must be selected in such a way that the sample intended for testing is heated evenly.
At these moments, the heat flows will be equal and the heat transfer ratio will be determined as the difference in the rates of temperature fluctuations. Sometimes during these studies, the source of indirect heating of the test and comparative sample. Additional thermal loads may be created on one of the samples in comparison with the second sample.
How to tell the difference?
Most often you can distinguish by:
without the use of any tools or equipment.
But there are situations when, for accuracy, it is necessary to use :
- reagents,
- tools,
- devices.
Before assessing the scrap that you are going to take to the collection point, you need to clean it of dirt, otherwise you won’t be able to accurately determine it by eye.
By color
Both metals, although to varying degrees, can develop a patina .
Therefore, do not forget to clean the scrap well.
If an object has been in the open air or in water for a long time, the patina layer is difficult to remove.
Sometimes it will be justified to purchase a special cleaning product .
It is advisable to inspect the scrap under a powerful white light.
This implies that one can view either under the sun on a fine day or under a bright fluorescent lamp . Incandescent lamp is not suitable.
Pure copper will have a reddish-brown tint, sometimes with a pink tint. Keep in mind that brass can be red or orange. This type is commonly used for decorations and water pipes.
If the material has an orange, yellow or golden tint, you can be almost sure that it is brass.
If you are engaged in the collection and delivery of scrap metal, then it will be useful for you to know the prices for ferrous scrap metal. If you don’t know where to find ferrous metals, then read this article. Not sure which metal detector model to choose? Check out the review of popular models https://rcycle.net/metally/cvetnye/metalloiskateli-vidy-modelj-i-ceny.
It can also be light golden , pale yellow , and even off-white , but it is very rare for metal detectors, since such an alloy is difficult to process, and it is used mainly in jewelry.
The best recommendation is to carry an item that you are absolutely sure is made of pure copper . You will be able to compare it with the crowbar you found. Most often this method works well.
By sound
Another method that does not require special skills or equipment. You can learn to distinguish metals by sound after a short training. Hit the object with something metal. If it is made of copper, the sound will be muffled and low . This happens because the metal is soft.
Visual inspection and testing for sound and hardness are usually sufficient for field determination.
On the contrary, brass will produce a ringing and high-pitched sound when struck. The second most important inspection method for those who deal with scrap metal is after visual assessment in the light. But this method is justified only with large and voluminous objects - you need something to make a sound.
By hardness
Copper, as mentioned above, is a soft metal. Brass was specifically created to increase the hardness of copper while maintaining some of its other characteristics. Therefore, when damage is caused to scrap, copper will be the material that is more easily deformed . Brass, on the other hand, can withstand impacts .
Which thermal conductivity measurement method is best for your material?
There are methods for measuring thermal conductivity such as LFA, GHP, HFM and TCT. They differ from each other in the sizes and geometric parameters of the samples used to test the thermal conductivity of metals.
These abbreviations can be deciphered as:
- GHP (hot guard zone method);
- HFM (heat flow method);
- TCT (hot wire method).
The above methods are used to determine the coefficients of various metals and their alloys. At the same time, using these methods, they study other materials, for example, mineral ceramics or refractory materials.
The metal samples on which the research is carried out have overall dimensions of 12.7 × 12.7 × 2.
How to determine what we have in front of us: brass or copper, their main differences
Anyone who searches for and sells non-ferrous metal sometimes has doubts about the type of scrap and, accordingly, its true value upon delivery.
Copper is a non-ferrous metal, and brass is an alloy that is typically 70% copper, so it often resembles it.
A mistake can be quite costly. For copper at collection points they give 285-300 rubles, for brass - about 150 . There are many ways to find out what kind of metal we see - copper or brass, and we will tell you how to distinguish them from each other in this article.
A little about thermal conductivity
In physics, thermal conductivity is understood as the movement of energy in an object from more heated small particles to less heated ones. Thanks to this process, the temperature of the object in question as a whole is equalized. The magnitude of the ability to conduct heat is characterized by the thermal conductivity coefficient. This parameter is equal to the amount of heat that a material 1 meter thick passes through a surface area of 1 m2 for one second at a unit temperature difference.
| Material | Thermal conductivity coefficient, W/(m*K) |
| Silver | 428 |
| Copper | 394 |
| Aluminum | 220 |
| Iron | 74 |
| Steel | 45 |
| Lead | 35 |
| Brick | 0,77 |
Copper has a thermal conductivity coefficient of 394 W/(m*K) at temperatures from 20 to 100 °C. Only silver can compete with it. And for steel and iron this figure is 9 and 6 times lower, respectively (see table). It is worth noting that the thermal conductivity of products made from copper largely depends on impurities (however, this also applies to other metals). For example, the rate of heat conduction decreases if substances such as:
- iron;
- arsenic;
- oxygen;
- selenium;
- aluminum;
- antimony;
- phosphorus;
- sulfur.
Copper wire
If you add zinc to copper, you get brass, which has a much lower thermal conductivity coefficient. At the same time, adding other substances to copper can significantly reduce the cost of finished products and give them characteristics such as strength and wear resistance. For example, brass is characterized by higher technological, mechanical and anti-friction properties.
Since high thermal conductivity is characterized by rapid distribution of heating energy throughout the entire object, copper is widely used in heat exchange systems. At the moment, radiators and tubes for refrigerators, vacuum units and cars are made from it for rapid heat removal. Copper elements are also used in heating installations, but for heating.
Copper heating radiator
In order to maintain the thermal conductivity of the metal at a high level (and therefore make the operation of copper devices as efficient as possible), forced airflow by fans is used in all heat exchange systems. This decision is due to the fact that as the temperature of the environment increases, the thermal conductivity of any material decreases significantly, because heat transfer slows down.
Thermal conductivity of aluminum and copper - which metal is better?
The thermal conductivity of aluminum and copper is different - for the first it is 1.5 times less than for the second. For aluminum, this parameter is 202–236 W/(m * K) and is quite high compared to other metals, but lower than that of gold, copper, and silver. The scope of application of aluminum and copper, where high thermal conductivity is required, depends on a number of other properties of these materials.
Aluminum is not inferior to copper in anti-corrosion properties and is superior in the following indicators:
- the density (specific gravity) of aluminum is 3 times less;
- the cost is 3.5 times lower.
A similar product, but made of aluminum, is much lighter than copper. Since the weight of metal required is 3 times less, and its price is 3.5 times lower, an aluminum part can be about 10 times cheaper. Thanks to this and its high thermal conductivity, aluminum is widely used in the production of tableware and food foil for ovens. Since this metal is soft, it is not used in its pure form - its alloys are mainly common (the most famous is duralumin).
In various heat exchangers, the main thing is the rate of release of excess energy into the environment. This problem is solved by intensively blowing the radiator using a fan. At the same time, the lower thermal conductivity of aluminum practically does not affect the quality of cooling, and equipment and devices are much lighter and cheaper (for example, computers and household appliances). Recently, there has been a tendency in production to replace copper tubes in air conditioning systems with aluminum ones.
Copper is practically irreplaceable in the radio industry and electronics as a conductive material. Due to its high ductility, it can be used to draw wire with a diameter of up to 0.005 mm and to make other very thin conductive connections used for electronic devices. Higher conductivity than aluminum ensures minimal losses and less heating of radio elements. Thermal conductivity allows you to effectively remove the heat generated during operation to the external elements of devices - housing, supply contacts (for example, microcircuits, modern microprocessors).
Copper templates are used in welding when it is necessary to deposit the desired shape on a steel part. High thermal conductivity will not allow the copper template to connect to the welded metal. Aluminum cannot be used in such cases, since there is a high probability of it melting or burning. Copper is also used in carbon arc welding - a rod made of this material serves as a non-consumable cathode.
Pros and cons of aluminum radiators
By comparing the strengths and weaknesses of devices, you can understand their main differences. After all, the difference between copper and aluminum radiators lies in their main characteristics. What is considered an objective advantage for one person turns out to be a serious disadvantage for another. Just look at the pros and cons of aluminum products and you will understand the difference between them.
Let's start with the positive aspects of aluminum as a material for the manufacture of car heater radiators.
- Price. While cost was considered a disadvantage of copper radiators, here it is a serious advantage. If we compare the price tags for both products, aluminum ones will win by about 2 times. Much depends on the manufacturer, but still the difference in cost remains significant. The buyer can save a lot. Because of this, aluminum units have such a large audience.
- Heat dissipation. Provided that the number of plates is increased, that is, the cooling area becomes larger, aluminum will not be much inferior to copper in terms of heat transfer. Therefore, in this component they are practically the same. But remember that aluminum ones are cheaper.
- Range. A huge proportion of modern cars that have been produced over the past few years are equipped with aluminum units from the factory. Because of this, the number of their analogues and original spare parts offered by different manufacturers is growing. The copper versions have a more modest choice.
We're done with the benefits. Let's move on to the other side of the coin. Aluminum is not doing so well. The stated advantages are beyond doubt. But still, motorists make a choice in favor of copper after they have studied the main disadvantages of the design option under consideration.
Therefore, the disadvantages should definitely be pointed out. This clearly shows the differences between the elements. The main disadvantages include:
- Thermal conductivity indicators. This is a very important drawback that literally negates all the objective positive qualities of the devices. If the driver needs to get the most efficient radiator so that the heating system works efficiently and fully warms up the interior, he will not look in the direction of aluminum.
- Repairability
Approximately the same conclusions can be drawn regarding these devices, which are made from two different materials.
Disadvantages of the high thermal conductivity of copper and its alloys
Copper has a much higher cost than brass or aluminum. At the same time, this metal has its disadvantages, which are directly related to its advantages. High thermal conductivity leads to the need to create special conditions during cutting, welding and soldering of copper elements. Since copper elements need to be heated much more concentrated compared to steel. Also, preliminary and concomitant heating of the part is often required.
Don’t forget that copper pipes require careful insulation if they make up the main line or distribution of the heating system. Which leads to an increase in the cost of network installation compared to options when other materials are used.
Example of thermal insulation of copper pipes
Difficulties also arise with gas welding of copper: this process will require more powerful torches. When welding metal 8–10 mm thick, two or three torches will be required. While one torch is used for welding, the other is heating the part. In general, welding work with copper requires increased costs for consumables.
It should also be said about the need to use special tools. So, to cut brass and bronze up to 15 cm thick, you will need a cutter capable of working with high-chromium steel 30 cm thick. Moreover, the same tool is enough to work with pure copper only 5 cm thick.
Plasma cutting of copper
Is it possible to increase the thermal conductivity of copper?
Copper is widely used in the creation of microcircuits for electronic devices and is designed to remove heat from parts heated by electric current. When trying to increase the speed of modern computers, developers were faced with the problem of cooling processors and other parts. One of the solutions was to split the processor into several cores. However, this method of combating overheating has exhausted itself, and now it is necessary to look for new conductors with higher thermal and electrical conductivity.
One solution to this problem is the recently discovered element graphene. Thanks to graphene deposition, the thermal conductivity of the copper element increases by 25%. However, the invention is still at the development level.