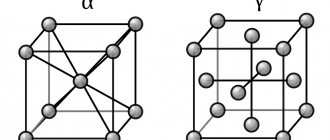
In the metallurgical industry, one of the main areas is the casting of metals and their alloys due to the low cost and relative simplicity of the process. Molds with any shape and various dimensions can be cast, from small to large; It is suitable for both mass and customized production.
Casting is one of the oldest areas of working with metals, and begins around the Bronze Age: 7-3 millennium BC. e. Since then, many materials have been discovered, leading to advancements in technology and increased demands on the foundry industry.
Nowadays, there are many directions and types of casting, differing in technological process. One thing remains unchanged - the physical property of metals to pass from a solid to a liquid state, and it is important to know at what temperature the melting of different types of metals and their alloys begins.
Metal melting process
This process refers to the transition of a substance from a solid to a liquid state. When the melting point is reached, the metal can be in either a solid or liquid state; further increase will lead to the complete transition of the material into a liquid.
The same thing happens during solidification - when the melting point is reached, the substance will begin to transition from a liquid to a solid state, and the temperature will not change until complete crystallization.
It should be remembered that this rule applies only to pure metal. Alloys do not have a clear temperature boundary and undergo state transitions in a certain range:
- Solidus is the temperature line at which the most fusible component of the alloy begins to melt.
- Liquidus is the final melting point of all components, below which the first alloy crystals begin to appear.
It is impossible to accurately measure the melting point of such substances; the point of transition of states is indicated by a numerical interval.
Depending on the temperature at which metals begin to melt, they are usually divided into:
- Low-melting, up to 600 °C. These include tin, zinc, lead and others.
- Medium melting, up to 1600 °C. Most common alloys, and metals such as gold, silver, copper, iron, aluminum.
- Refractory, over 1600 °C. Titanium, molybdenum, tungsten, chromium.
There is also a boiling point - the point at which the molten metal begins to transition into a gaseous state. This is a very high temperature, typically 2 times the melting point.
Effect of pressure
The melting temperature and the equal solidification temperature depend on pressure, increasing with its increase. This is due to the fact that with increasing pressure the atoms come closer to each other, and in order to destroy the crystal lattice they need to be moved away. At increased pressure, greater thermal energy is required and the corresponding melting temperature increases.
There are exceptions when the temperature required to transform into a liquid state decreases with increased pressure. Such substances include ice, bismuth, germanium and antimony.
Security measures
It is impossible to get lead to boil at home, since the temperature must be extremely high. One way or another, the volatility of this metal increases noticeably already at 700 degrees.
When temperatures rise, people nearby may suffer from the adverse effects of material fumes .
If there is no need, there is no need to bring the lead to “redness”. Molten lead can cause harm in the following ways:
- If it gets on the surface of the skin, it can cause severe burns, since the melting point of lead is quite high.
- Drops of metal quickly burn through clothing.
- If hot metal comes into contact with flammable objects and materials, it can easily cause a fire.
Read also: Welding copper with brass
You should also avoid getting liquid into hot metal. Otherwise, a fountain of hot splashes may appear, which can cause a lot of problems.
Lead should be melted in the fresh air or in a room with good ventilation. It is not advisable to avoid using protective equipment . A respirator or ordinary gauze can protect the lungs from metal dust.
Melting point table
It is important for anyone involved in the metallurgical industry, whether a welder, foundry worker, smelter or jeweler, to know the temperatures at which the materials they work with melt. The table below shows the melting points of the most common substances.
Read also: What can be cast from lead
Table of melting temperatures of metals and alloys
| Name | T pl, °C |
| Aluminum | 660,4 |
| Copper | 1084,5 |
| Tin | 231,9 |
| Zinc | 419,5 |
| Tungsten | 3420 |
| Nickel | 1455 |
| Silver | 960 |
| Gold | 1064,4 |
| Platinum | 1768 |
| Titanium | 1668 |
| Duralumin | 650 |
| Carbon steel | 1100−1500 |
| Cast iron | 1110−1400 |
| Iron | 1539 |
| Mercury | -38,9 |
| Cupronickel | 1170 |
| Zirconium | 3530 |
| Silicon | 1414 |
| Nichrome | 1400 |
| Bismuth | 271,4 |
| Germanium | 938,2 |
| Tin | 1300−1500 |
| Bronze | 930−1140 |
| Cobalt | 1494 |
| Potassium | 63 |
| Sodium | 93,8 |
| Brass | 1000 |
| Magnesium | 650 |
| Manganese | 1246 |
| Chromium | 2130 |
| Molybdenum | 2890 |
| Lead | 327,4 |
| Beryllium | 1287 |
| Will win | 3150 |
| Fechral | 1460 |
| Antimony | 630,6 |
| titanium carbide | 3150 |
| zirconium carbide | 3530 |
| Gallium | 29,76 |
In addition to the melting table, there are many other supporting materials. For example, the answer to the question what is the boiling point of iron lies in the table of boiling substances. In addition to boiling, metals have a number of other physical properties, such as strength.
Bibliography
- Kawakami K., Kitagawa T., Miyashita Y. et al. II Nippon Kokan Technical Report.Overseas. 1982. V. 36. P. 26...27.
- SchreweH. II Verlag Stakleisen mbH. Dusseldorf, 1987. S. 104.
- Deuxieme Conference Mondial des Founders a models perdus. Dusseldorf, 1…4 June, 1960.
- AymardJ. P., DetrezP. IIFouderie 330. Janvier, 1974. P. 11...24.
- Hirai M., Kanamru K., Mori H. IITetsu to Hagane 52 (1969). P. 85.
- Roeser Wm. R, Wensel H. T. Freezing Temperatures of High-Purity Iron and Some Steels // Journal of Research of the National Bureau of Standards. 1941. V. 26. P. 273…287.
- Kagava A., Okamota T. Influence of alloying elements on temperature and composition for peritectic reaction in plain carbon steel // Material science and technology. October 1986. V. 2. No. 10. P. 997...1008.
- Andrews KW Solidification ranges of steel // A note submitted to the alloy phase diagram date Committee of the Metals Society, 1981. P. 1…8.
- WolfM. //Zurich, 1982. S. 37...49.
- Howe AA II Ironmaking and Steelmaking. 1988. V. 16. No. 3. P. 134…142.
- Jerkontoret.//Stockholm, 1977. P. 117.
- Schiirmann E., Schweinichen JV, Volker R. ua II Giesserei-Forschung 39, Jahrgang 1987. H. 4. S. 133…136.
- SugdenA. A. V., Bhadeshia HKDH II Material science and technology. October 1989. V. 5. No. 10. P. 977…984.
You can ask a question you are interested in or write a comment on this article here. Write to us and we will definitely answer.
Strength of metals
In addition to the ability to transition from a solid to a liquid state, one of the important properties of a material is its strength - the ability of a solid body to resist destruction and irreversible changes in shape. The main indicator of strength is the resistance that occurs when a pre-annealed workpiece breaks. The concept of strength does not apply to mercury because it is in a liquid state. The designation of strength is accepted in MPa - Mega Pascals.
There are the following strength groups of metals:
- Fragile. Their resistance does not exceed 50MPa. These include tin, lead, soft-alkaline metals
- Durable, 50−500 MPa. Copper, aluminum, iron, titanium. Materials of this group are the basis of many structural alloys.
- High strength, over 500 MPa. For example, molybdenum and tungsten.
Metal strength table
| Metal | Resistance, MPa |
| Copper | 200−250 |
| Silver | 150 |
| Tin | 27 |
| Gold | 120 |
| Lead | 18 |
| Zinc | 120−140 |
| Magnesium | 120−200 |
| Iron | 200−300 |
| Aluminum | 120 |
| Titanium | 580 |
The most common alloys in everyday life
As can be seen from the table, the melting points of elements vary greatly even among materials commonly found in everyday life.
Thus, the minimum melting point of mercury is -38.9 °C, so at room temperature it is already in a liquid state. This explains why household thermometers have a lower mark of -39 degrees Celsius: below this indicator, mercury turns into a solid state.
The most common solders in household use contain a significant percentage of tin, which has a melting point of 231.9 °C, so most solders melt at the operating temperature of the soldering iron 250−400 °C.
In addition, there are low-melting solders with a lower melt limit, up to 30 °C, and are used when overheating of the materials being soldered is dangerous. For these purposes, there are solders with bismuth, and the melting of these materials lies in the range from 29.7 - 120 °C.
Melting of high-carbon materials, depending on alloying components, ranges from 1100 to 1500 °C.
The melting points of metals and their alloys are in a very wide temperature range, from very low temperatures (mercury) to several thousand degrees. Knowledge of these indicators, as well as other physical properties, is very important for people who work in the metallurgical field. For example, knowledge of the temperature at which gold and other metals melt will be useful to jewelers, foundries and smelters.
Metals melt, as a rule, at a very high temperature, which can reach more than 3 thousand degrees. Although some of them can be melted at home, such as lead or tin. But mercury is melted at a temperature of minus 39 degrees. This cannot be achieved at home. Melting point is one of the important indicators of the production of not only the metal itself, but also its alloys. When smelting raw materials, specialists take into account other physical and chemical properties of the ore and metal.
Read also: Why does stainless steel rust after welding?
Historical information
The chemical element has been known to people since ancient times. One of the first methods of metal extraction mastered by man was the smelting of lead. The first archaeological finds confirming this were lead beads found from the times of Çatalhöyük (modern Turkey). The items date back to 6400 BC.
The oldest lead figurine of a girl in long clothes was excavated in Egypt. It dates back to the time of the first dynasty of the pharaohs (3000 BC).
Lead pipes made up the ancient Roman water supply system. In the Ancient Roman Empire, up to 80 thousand tons of this metal were smelted annually. In Rus', since ancient times, lead has been used as roofing for cathedrals and churches.
Since time immemorial, the low melting point of lead has made it possible to obtain the metal and manufacture products of any shape from it.
Note! Over the course of 20 years, the Industrial Revolution since 1840 has raised the volume of annual lead smelting in the world from 100 to 250 thousand tons per year.
Iron and its properties
Iron is a chemical element that is number 26 on the periodic table. It is one of the most abundant elements in the entire solar system. According to research materials, the Earth's core contains approximately 79−85% of this substance . There is also a large amount of it in the earth's crust, but it is inferior to aluminum.
In its pure form, the metal is white with a slightly silvery tint. It is plastic, but the impurities present in it can determine its physical properties. Reacts to a magnet.
Iron is present in water. In river waters its concentration is approximately 2 mg/l of metal. In sea water its content can be a hundred or even a thousand times lower.
Iron oxide is the main form that is mined and found in nature. Iron oxide can be located in the uppermost part of the earth's crust and be a component of sedimentary formations.
An element in twenty-sixth place on the periodic table can have several oxidation states. It is they who determine its geochemical feature of being in a certain environment. In the Earth's core, the metal is present in a neutral form.
Methods for getting rid of oxide
When exposed to air, lead products become covered with an oxide film. This is the result of the ionic interaction of oxygen and lead atoms. The oxide becomes not only protection against an aggressive environment, but also a barrier to electric current.
Important! Mechanical cleaning will not bring the desired result. The film will recover quite quickly. Sunflower oil, graphite grease or varnish can help get rid of oxides.
At home, the product is placed in a vessel with sunflower oil for about five minutes. After which it is removed from the vessel and allowed to dry.
In industrial conditions, graphite lubricant is used. The lead surface treated with the product retains its shiny appearance for a long time.
Mining
There are several ores containing iron. However, the following are mainly used as raw materials for iron production in industry:
- magnesite ore;
- goethite ore;
- hematite ore.
And also the following types of ore are often found:
- lellingitis;
- siderite;
- marcasite;
- ilmenite;
- is violent.
There is also a mineral called melanterite . It is used primarily in the pharmaceutical industry. It consists of green, fragile crystals with a glassy sheen. Medicines containing ferum are produced from it.
The main deposit of this metal is South America, namely Brazil.
Safety precautions
The boiling point of lead is very high (1751 °C), and it is impossible to achieve it at home. Nevertheless, its volatility becomes quite high already at 700 °C. If the melting point is significantly exceeded, nearby people may be adversely affected by its fumes. If there is no significant technological need to overheat the melt, it should not be brought to a reddish tint. This is precisely what indicates that the melting point of lead was exceeded.
Molten lead can cause serious burns if it comes into contact with the skin. Its drops burn through clothing and, if they come into contact with flammable materials, can cause a fire and, as a consequence, a fire, so you should work carefully. Water should not get into molten lead. This causes a fountain of silvery spray, which, if it gets into the eyes, can cause extremely undesirable consequences.
Work should be done in a well-ventilated area or outdoors. Do not neglect personal protective equipment. A respirator or even a cotton-gauze bandage will protect your respiratory tract from lead dust. Small doses of lead in the body may not immediately cause consequences. However, this material tends to accumulate over a lifetime and cause poisoning if permissible doses are exceeded.
Source
Iron melting and required temperature
The melting point of a metal is the minimum temperature at which it changes from solid to liquid. At the same time, it remains practically unchanged in volume.
Metal can be produced from ore in various ways, but the most basic of them is blast furnace . In addition to blast furnace, iron smelting is also used by roasting crushed ore with an admixture of clay. From the resulting mixture, pellets are formed, which are processed in a furnace followed by reduction with hydrogen. Next, the iron is melted in an electric furnace.
The melting point of iron is very high. For a technically pure element it is +1539 °C. This substance contains an impurity - Sulfur, which can only be extracted in liquid form. Without impurities, pure material is obtained by electrolysis of metal salts.
Receipt
How to solder a wire without a soldering iron
The raw materials for lead extraction are rocks that include helenite. The heavy metal smelting process consists of several phases. From the initial raw material, a concentrate containing from 40 to 70 percent plumbum is isolated by flotation. Next, manufacturers take different paths.
One of the ways to transform the product into werkbley (blank lead) is smelting using the regeneration method. Another method is that the metal is restored from the oxide by melting the raw material in a water jacket heater.
The resulting werkley containing 90% lead is purified from copper. Then arsenic and antimony are removed by alkaline refining. Then silver and zinc are isolated. The effects of magnesium and calcium exclude bismuth. As a result, lead with a purity of 99.8% is obtained.
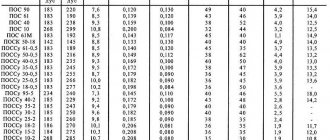
Global lead production based on research by international organizations for 2005
| Manufacturer country | Volume, kilotons |
| Countries of Europe | 2220 |
| China | 1430 |
| Russian Federation | 1120 |
| South Korea | 650 |
| Kazakhstan | 570 |
| Ukraine | 410 |
Classification of metals by melting point
Different metals can turn liquid at different temperatures. As a result, a certain classification is distinguished. They are divided as follows:
- Low-fusibility elements are those elements that can become liquid even at temperatures below 600 degrees. These include zinc, tin, lead, etc. They can be melted even at home - you just need to heat them up using a stove or soldering iron. Such types have found application in technology and electronics. They are used to connect metal elements and move electric current. Tin melts at 232 degrees, and zinc at 419 degrees.
- Medium-melting - elements that begin to melt at temperatures from six hundred to one thousand six hundred degrees. These elements are used mainly for building elements and metal structures, that is, when creating fittings, slabs and building blocks. This group includes: iron, copper, aluminum. The melting point of aluminum is relatively low and is 660 degrees. But iron begins to turn into a liquid state only at a temperature of 1539 degrees. It is one of the most common metals used in industry, especially in the automotive industry. However, iron is susceptible to corrosion, that is, rust, so it requires special surface treatment. It must be coated with paint or drying oil, and moisture must not be allowed to enter.
- Refractory are materials that melt and become liquid at temperatures above 1600 degrees. This group includes tungsten, titanium, platinum, chromium, etc. They are used in the nuclear industry and for some machine parts. They can be used for melting other metals, making high-voltage wires or wire. Platinum can be melted at 1769 degrees, and tungsten at 3420 °C.
Read also: DIY water level sensors
The only element that is in a liquid state under normal conditions is mercury. Its melting point is minus 39 degrees and its vapors are poisonous, so it is used only in laboratories and closed containers.
Almost all metals are solids under normal conditions. But at certain temperatures they can change their state of aggregation and become liquid. Let's find out what is the highest melting point of metal? Which is the lowest?
Application areas of lead alloys
Lead compounds are divided into high-alloy and low-alloy alloys. The former are formed by adding a large number of chemical elements that provide high strength, abrasion resistance and low shrinkage at a lower melting point.
Low alloy lead compounds are produced by small inclusions of substances such as tin, antimony, copper and cadmium. This achieves increased resistance of the alloy to corrosion processes in a polluted atmosphere, inorganic acidic environment.
The alloys are used in acid and alkaline batteries, as shells for both high-power and low-voltage cables. Compounds of antimony or copper with lead are used for the production of pipelines, sheet lining of various devices and protective mats against radiation damage.