Basic concept of amphotericity
What metals and non-metals are is not difficult to understand. Metals have reducing properties and give up electrons in a chemical reaction. At the same time, metal hydroxides are bases. Nonmetals, on the other hand, are oxidizing agents and take away electrons. Non-metal hydroxides are acids.
Source
Amphoteric compounds can exhibit both oxidizing and reducing properties depending on the reaction medium. Hydroxides of such atoms can act as acids or bases.
Application area
All of the above characteristics have allowed TsAM to become widespread in various types of production. Among them the following stand out:
- TsAMs are most widely used in the automotive industry. They are used to produce thin-walled carburetor and pump housings, radiator grilles and hydraulic brake elements.
- The bearing industry uses the alloy as a material for the manufacture of plain bearings and monometallic liners.
- In textile production, due to the ability of alloys to convey complex shades well, zippers, snaps and buttons are made.
- In the food industry, the alloy can be found as a material for parts of refrigerators, dishwashers and other household appliances.
- The trigger mechanism of small arms is produced from TsAM.
- Door fittings: handles, hinges, lock elements, etc.
- Fishing gear: reels, fishing rod elements, etc.
- You can increasingly find TsAM in watch movements.
- All kinds of souvenirs and toys.
Location of amphoteric elements in the periodic table
In the periodic table, the position of a particular atom provides a significant part of the information about the structure of the atom of this element and its chemical properties. This system is called periodic because a certain quality of elements is repeated in different periods (horizontal lines) and groups (vertical columns). So, the entire first group is alkali metals, the seventh is halogens (non-metals), the eighth is inert gases. But this is typical only for the main subgroup. The side group contains amphoteric elements.
Atomic structure of amphoteric elements
The peculiarity of the chemical properties of amphoteric elements is associated with the structure of their atoms. They pre-fill the s-sublevel, because of this, the d-sublevel is always unfilled. All representatives of side subgroups are p- or d-elements. Under various conditions, a jump of electrons from sublevels and an increase in unpaired electrons can occur.
Table. Atomic structure of some amphoteric elements
Some of them are characterized by electron leakage. This is a state in which an electron from the last level jumps to the next. For this reason, the s-electron turns out to be unpaired.
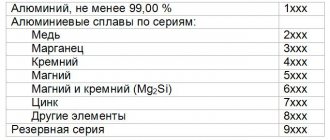
Aluminum casting alloys series 7хх.х
According to the American classification, these alloys belong to the 7xxx.x series. They have medium to high strength properties. Annealing ensures good dimensional stability. The eutectic point of alloys of this group is high, which is favorable for parts that are subject to soldering. These alloys have good machinability. They are characterized by high corrosion resistance with some tendency to stress corrosion. They are not recommended for use at elevated temperatures. The strength properties of these alloys increase at room temperature for several weeks after casting as a result of strengthening through the mechanism of release of the secondary phase. This process continues after several weeks, but at a decreasing speed. Alloys 707.0, 771.0 and 772.0 can be treated with T6 and T7 heat treatments.
Representatives of amphoteric elements
All elements of side groups are amphoteric and exhibit similar chemical properties. The three most common elements in nature are Al, Zn and Cr.
Zinc as an amphoteric element
Zinc is a relatively soft, light gray metal. It is one of the most common amphoteric elements. In nature, zinc is found in 66 minerals, the most common are presented in the table.
Table. Minerals containing Zn
Zinc is a d-element.
1s22s22p63s23p63d104s2
The chemical properties of zinc are due to the presence of an unfilled p-habitat. An electron jumps from the s-sublevel, due to which two unpaired electrons appear: Zn* 1s22s22p63s23p63d104s14p1.
Aluminum as an amphoteric element
Al is the most common element not only among metals, but also in the entire periodic table. It ranks 3rd after oxygen (O2) and silicon (Si).
It is a soft, silver-gray substance with a low melting point. It occurs in nature both in the form of minerals and in the form of nuggets. It is an admixture of many minerals.
The most common minerals containing Al are:
- Augite ((Ca,Na)(Mg,Fe,Al,Ti)(Si,Al)2O6)
- Bauxite (Al2O3xH2O)
- Nepheline (Eliolite) ((NaK)AlSiO4)
- Alunite (K2SO4Al2(SO4)3 4Al(OH)3)
- Sillimanite ((Al2O3)(SiO2))
- Corundum (Al2O3)
The latter mineral has a different color depending on the impurities. It is used in jewelry and is considered a semi-precious stone.
Its atom contains 13 electrons distributed over 3 electronic levels: 1s22s22p63s23p1. This is a p-element in which an electron can transition from the s-sublevel to a free p-orbital. Due to this, the metal acquires 3 unpaired electrons: Al* 1s22s22p63s13p2
Galfan coated steel processing
Molding
Typically, bare steel and galvanized steel can be formed using the same methods without significantly changing process conditions. Due to slight differences in surface properties, it is sometimes necessary to make minor changes, for example to lubrication, tool geometry or clamping force. The advantages of metal coating include its lubricating effect, which is effective at low and moderate surface pressure during the molding process.
The laminar microstructure of Galfan coating is ideal for roll forming, advanced deep drawing, profiling and bending. The thinnest intermetallic connecting layer of Galfan coating with steel exhibits tremendous resistance to cracking. These two properties make Galfan ideal for complex molding applications.
The results of forming metal-coated steel depend on factors such as feature geometry, steel grade, type of metal coating, thickness, surface quality and protection, and forming tools.
Welding
In general, metal-coated steel products can be welded using a variety of methods, including resistance welding, laser welding, and arc welding. When welding recommendations are followed, the mechanical properties of the welds are no different from those of uncoated steel. Welding of steel products with zinc-aluminum (ZA) coating is carried out with the same parameters as welding of galvanized (Z) steel. Reducing the coating thickness through the use of Galfan zinc-aluminum alloy makes it possible to reduce the welding current and extend the service life of the electrodes.
Metal coated steel is most often processed using resistance welding processes such as spot welding, which provide excellent results. The beneficial anti-corrosion properties of zinc-based coatings are generally localized to the area of properly performed spot welding. Due to the reduced contact resistance of coated steel, spot welding requires slightly higher current and force on the electrodes compared to uncoated steel. Likewise, the welding current increases slightly with increasing coating thickness. Therefore, it is not recommended to weld steel with an excessively thick coating, which reduces the suitability of the material for welding and shortens the service life of welding electrodes.
Metal-coated steel is also ideal for laser welding, characterized by narrow (only a few mm) seams and low heat input. The use of any fusion welding method dictates the need to limit to a minimum the area of the metal-coated steel sheet subject to heating, and therefore the heat input. Like a scratched surface, the narrow weld area is protected from cathodic corrosion due to the protective properties of the zinc-based coating. However, after fusion welding, it is recommended to paint or apply another suitable protective coating to the weld areas.
Properties of the metals Al and Zn as simple substances
Zinc is a fairly dense metal. It retains its qualities in a small temperature range: at low temperatures (up to -30) it becomes brittle, at temperatures above 1000 C it is very plastic. This is used in metallurgy by rolling zinc sheets a few millimeters thick (zinc foil). Some impurities dramatically increase the fragility of the metal, so purified material is used.
Al is a highly ductile light metal with a low melting point. It has high malleability and electrical conductivity.
In air it is covered with an oxide film and therefore practically does not corrode. Due to this, it is used in the manufacture of wires and machinery housings.
Cast aluminum alloy 771.0
Chemical composition
Alloy formula: 7Zn-0.9Mg-0.13Cr
Chemical composition:
- copper: 0.10% max;
- magnesium: 0.8-1.0%;
- manganese: 0.10% max;
- silicon: 0.15% max;
- iron: 0.15% max;
- chromium: 0.06-0.20%;
- zinc: 6.5-7.5%;
- Tin: 3.5% max;
- titanium: 0.10-0.20%;
- others: 0.05% each, 0.15% total max;
- aluminum: the rest.
Properties: mechanical and physical
Typical mechanical properties (T5):
- tensile strength: 290 MPa;
- yield strength: 260 MPa;
- relative elongation: 1.5%;
- modulus of elasticity: 71.0 GPa.
Physical properties:
- density: 2.823 g/cm3;
- liquidus temperature: 645 ºС;
- solidus temperature: 605 ºС.
Heat treatment
This alloy can be heat treated to T2, T5, T51, T52 and T71 states
Heat treatment for T5 condition:
- exposure at 180 ºС for 3-5 hours;
- cooling outside the oven in still air.
Heat treatment for condition T51:
- exposure at 205 ºС for 6 hours;
- cooling outside the oven in still air.
Heat treatment for T6 condition:
- exposure at 580-595 ºС for 6 hours;
- cooling outside the oven in still air to room temperature;
- aging by exposure for 3 hours at a temperature of 130 ºС;
- cooling in still air.
Mechanical restoration
Alloy 771.0 in T5 condition has good stability and machinability. It can be milled 5 times faster and drilled 2 times faster than alloys such as 356.0 and 319.0.
Production of aluminum and zinc
The main method of obtaining metals is their isolation from ore. For this purpose, the rock richest in metal is used. Aluminum is obtained from bauxite. This process consists of three stages:
- Rock mining;
- Enrichment (increasing metal concentration due to purification from impurities);
- Isolation of a pure substance by electrolysis.
Zinc is produced by several methods - electrolytic (as well as Al) and pyrometallurgical. The second method is based on the reduction of zinc from its oxide with carbon or carbon monoxide II (carbon monoxide):
ZnO + C ⇄ Zn + CO
ZnO + CO ⇄ Zn + CO2
The advantage of this method is that the products of the first reaction can be used in the second, which reduces the amount of emissions into the atmosphere.
Zinc production
Metal mining
Zinc as a native metal does not occur in nature. It is mined from polymetallic ores containing 1–4% metal in the form of sulfide, as well as copper, lead, gold, silver, bismuth and cadmium. The ores are enriched by selective flotation and zinc concentrates (50–60% Zn) are obtained.
Zinc concentrates are fired in furnaces. Zinc sulfide is converted to ZnO oxide. This releases sulfur dioxide SO2, which is used in the production of sulfuric acid.
Metal production
There are two ways to obtain pure zinc from ZnO oxide.
The most ancient method is distillation. The fired concentrated composition is subjected to heat treatment to give it granularity and gas permeability.
The concentrate is then reduced with coke or coal at a temperature of 1200–1300 °C. The process produces metal vapors, which are condensed and poured into molds. The liquid metal is separated from iron and lead at a temperature of 500 °C. This achieves zinc with a purity of 98.7%.
Sometimes complex and expensive processing of zinc by rectification is used - separation of mixtures due to the exchange of heat between steam and liquid. This cleaning allows you to obtain metal with a purity of 99.995% and remove cadmium.
The second method of producing zinc is electrolytic. The calcined concentrate is treated with sulfuric acid. The finished sulfate solution is cleaned of impurities, after which it is subjected to electrolysis in lead baths. Zinc deposits on aluminum cathodes. The resulting metal is removed from the baths and melted in induction furnaces. After this, electrolytic zinc with a purity of 99.95% is obtained.
Metal casting
Hot zinc is a liquid and flowing metal. Thanks to these properties, it is easily filled into molds.
Impurities affect the amount of surface tension of the zinc. The technological properties of the metal can be improved by adding small amounts of lithium, magnesium, tin, calcium, lead or bismuth.
The higher the overheating temperature of zinc, the better it fills molds. When casting metal into cast iron molds, its volume decreases by 1.6%. This makes it difficult to produce large and long zinc castings.
Chemical properties of aluminum and zinc
Both substances are capable of reacting like ordinary metals. There are also a number of specific reactions.
Interaction with non-metals
Both substances interact with nonmetals to form binary compounds – salts. As a rule, the reaction rate and conditions depend on the activity of the nonmetal. So, the reaction with oxygen is the formation of an oxide when heated with zinc:
2Zn + O2 = 2ZnO
with aluminum under normal conditions:
4Al + 3O2 = 2Al2O3
Aluminum oxide covers the product with a dense film (oxide film) and the access of oxygen is stopped, therefore, for a complete reaction it must be taken in powder.
Zn does not react with Br, N2, Si, C, H2.
Al does not react with H2 alone.
Interaction with metals
With reducing agents, both metals form alloys:
- Aluminides CuAl2, CrAl7, FeAl3
- Brass ZnCu
This is not a chemical reaction because there is no transfer of electrons or change in the chemical properties of the substances.
Interaction with acids and alkalis
Both aluminum and zinc react with acids under normal conditions to form salts:
8Al + 30HNO3 = 8Al(NO3)3 + 3N2O + 15H2O;
2Al + 6HCl = 2AlCl3 + 3H2;
Zn + 2HCl = ZnCl2 + H2;
Zn + H2SO4 = ZnSO4 + H2.
The result of a reaction with alkalis depends on the reaction conditions: if the reaction occurs in solution (in the presence of water), then complex salts are formed:
2Al + 2NaOH + 10H2O = 2Na[Al(H2O)2(OH)4] + 3H2;
Zn + 2NaOH + 2H2O = Na2[Zn(OH)4] + H2.
In an anhydrous environment (fusion), salts of metallic acids are formed:
Zn + 2KOH = K2ZnO2 + H2 (K2ZnO2 – potassium zincate);
2Al + 6KOH = 2KAlO2 + 2K2O + 3H2 (KAlO2 – potassium aluminate).
Interaction with water
Aluminum actively interacts with water if the oxide film is cleaned. The reaction must be carried out quickly, since a film is formed almost instantly:
2Al + 6H2O = 2Al(OH)3 + 3H2;
Zn reacts with water at very high temperatures (when incandescent to red):
Zn + H2O = ZnO + H2.
General preparation of aluminum surface.
The issue of degreasing metals (including aluminum) is discussed in the article.
The process of etching aluminum has its own characteristics. When etching a number of chemical processes occur on the surface of aluminum. First, the dissolution of aluminum oxide in the cleanest places with the release of hydrogen and the formation of aluminates. Next, the dissolution of aluminum metal will occur with the formation of the same products, and the dissolution of the metal on the protrusions will proceed faster than in the recesses, due to which the surface will be leveled from large scratches. At the same time, surface etching will increase and micro-roughness will increase.
It is important that the etching of aluminum that has intermetallic compounds on the surface will proceed primarily along them (see article, paragraph 5.2).
When loading parts into the pickling bath, several degreasing factors also begin to work:
- Sodium hydroxide saponifies fats.
- The surfactant improves the wettability of parts and reduces the surface tension of the fat film.
- Actively released hydrogen mechanically tears the oil and fat film into the solution volume, where it is exposed to the action of the first two factors.
In some cases, during the general preparation of the surface of aluminum parts, it is justified to use a gentle etching solution that does not have a degreasing effect and is used for processing products with welded unsealed seams.
At the end of etching, a loose layer of sludge remains on the surface of the parts, consisting of products insoluble in alkali. The exact composition of the sludge depends on the alloying additives included in the aluminum alloys. Sludge removal occurs during the clarification operation. The composition of the solutions is different for wrought and cast alloys and is determined by the composition of the slurry. Wrought alloys are brightened in nitric acid, while aluminum foundry sludge, rich in silicon, does not dissolve in it. It requires adding hydrofluoric acid to the solution. Nitric acid does not act on aluminum, passivating it. Largely due to this, thin oxide layers remain on aluminum, preventing further metallization, but not preventing oxidation.
Zinc and aluminum oxides
ZnO is an oxide widely used in the chemical industry. It is used to obtain salts. In reaction with alkalis, complex salts are formed that are easily destroyed by acids.
Al2O3 – alumina. It has a very dense crystal lattice, which is why it practically does not react under normal conditions. At extremely high temperatures it reacts with alkalis:
Al2O3 + 2KOH = 2KAlO2 + H2O
May react with boiling acids to form complex salts.
Brass
It is a copper-based alloy with zinc as the main alloying component. The substance also contains tin (increases the mechanical strength of the alloy and resistance to oxidation in seawater), nickel and manganese (added for the same purpose), silicon (to improve weldability) and other additives. Among the advantages of the material is the high fluidity of molten metal, which allows it to be remelted and create products of even the most complex configurations. Brass is moderately viscous and has good thermal and electrical conductivity.
The only method for producing brass is casting. Copper and all necessary additives are melted in reverberatory furnaces or in refractory crucibles placed in an induction furnace with a powerful exhaust ventilation system.
Rolled brass is used in various industries and in everyday life. The alloy is used to make pipes, hardware, wire, machine parts and mechanisms, components of sliding bearings, building materials (for roofing, curtain facades), and pipeline fittings. It is used to create figurines and other decorative items and jewelry.
Application of aluminum and zinc
Al, as the most common element, is widely used in the chemical industry. It is capable of displacing reducing agents from compounds, therefore it is used for the production of metals. This method is called aluminothermy.
Due to the oxide film and low density, it is used in automobile, aircraft and rocket production to reduce the weight of the product. In construction, aluminum is used to make frames for high-rise buildings.
Zn is used to reduce corrosion of metal products – galvanizing. The powder of this metal is used to make oil paints with a metallic sheen. Also, the oxide serves as an antiseptic. Ointments based on zinc powder are used in the treatment of lichen and other infectious skin lesions.
Types of zinc alloys by purpose
According to their intended purpose, zinc alloys can be of several types:
- Deformable. 15% - aluminum, 5% - copper, more than 1% - magnesium. It is made in the form of sheets or rods. The properties are similar to brass.
- Foundries. They are made by adding 3-4% copper and aluminum to zinc, as well as 0.05% magnesium. They have good fluidity. Therefore, they are manufactured through injection molding or mold casting.
- Anti-friction. They contain 10% aluminum, 5% copper and 0.1% magnesium. Manufactured by injection molding. They have a low coefficient of friction and are used in the automotive industry.
- Solders. They are used for soldering aluminum parts. Usually include impurities - metals. This increases their strength.
- Typographic. They contain 7.5% aluminum, 2% magnesium and approximately 4% copper. Such alloys are very durable and cast well into molds.
- Protective. They contain no more than 1% aluminum and a tiny amount of silicon and magnesium. Resistant to corrosion even in damp environments. Therefore, such alloys are used as protective materials.
Zinc alloys have proven themselves well and are widely used. But when creating them, proportions must be accurately taken into account, otherwise poor quality material will be obtained.
Aluminum and zinc alloys
In metallurgy, they are practically not used in their pure form due to their high plasticity. In order to preserve the advantages of metals, but remove the disadvantages, alloying with other metals is carried out.
Aluminum alloys
Aluminum alloys are divided into two groups:
- Foundry (without maintaining plasticity);
- Structural (deformable).
Table. Characteristics of the main aluminum alloys
Zinc alloy in jewelry
Externally, zinc alloys resemble noble metals. Therefore, they are used in costume jewelry to create inexpensive jewelry. Zinc alloy jewelry looks expensive but is easy to create.
Is zinc alloy harmful? In fact, it has no effect on the human body. But, nevertheless, it is better to purchase good quality jewelry. Usually, a special alloy is used to create jewelry, which is called costume jewelry. The most commonly used is brass or an alloy with aluminum. Externally, such products resemble gold and silver.
Is zinc alloy harmful in jewelry? No, so you can safely buy products made from it. After all, if it had a negative impact, making jewelry from it would be prohibited.
Thus, zinc and zinc alloys are widely available. They are used in medicine, automotive industry and even in jewelry. These are high-quality and sustainable materials that practically do not change under the influence of environmental conditions.
Tags
non-ferrous metals Production of wire from Steel wire Steel wire Rod wire Steel wire Copper wire Brass wire welding wires for Production of wire from Nichrome wire nickel hard alloys are used to find equipment. Copper alloys of various alloys for Forms alloys almost a component of alloys for to alloys.its alloys.use non-ferrous metals
becometungstencircusminingcastironequipmentrolled metalbackworkcansteelotherstainless
Processing Features
The treatment is aimed at protecting metals from corrosion and contact with oxygen. To do this, they are coated with special paint and varnish coatings. There are 3 categories of protection products: paints, primers and universal compounds. The primer is most effective against atmospheric corrosion, and it also increases adhesion to the base of the part. It is applied to the surface before painting in one, or preferably several, layers.
It is important to correctly determine the type of primer, since the composition is different for different metals. For parts made of aluminum and its alloys, zinc-based primer compositions are suitable; Urethane paints are also suitable.
Copper alloys
Copper is one of the most popular materials in the production of electrical wiring, water pipes, and electronics. It is very refractory, conducts heat and electricity well, but is not strong enough and is difficult to cast. To eliminate this drawback, various impurities are added to the pure metal, which make the copper itself stronger. The resulting tin is not subject to corrosion and wear; thanks to these properties, its scope of application is incredibly wide.
Another common additive is zinc; a certain percentage of this metal makes it possible to obtain brass. In such a composition, the content of the alloying substance is very important; the higher the percentage of additive content, the harder the metal, but also the more susceptible to corrosive damage.
But the copper-nickel alloy is often used in the manufacture of jewelry; the additive gives a more pleasant color and durability to the products.
Where are they used?
Imagine a world without non-ferrous metals. Throw away your phone and computer, along with your car keys. Turn off the light - because current flows through wires made of non-ferrous metal. You will also have to throw away the gas and electric stoves, and cook over a fire or build a stove. Therefore, let us treat these different and so necessary metals with respect.
It is impossible to imagine the modern world without the use of non-ferrous metals.
Some of them are mined in millions of tons per year, others several tons per year. But all of them are absolutely necessary for modern industry and for us, consumers.
Electrical engineering, steel alloying, sensors, diodes, thermocouples, infrared optics, military-industrial complex.