Aluminum is the most abundant metal in the earth's crust. It belongs to the group of light metals, has a low density and melting point. At the same time, ductility and electrical conductivity are at a high level, which ensures its widespread use. So, let's find out what the specific melting point of aluminum and its alloys is (for example, in comparison with iron and lead), thermal and electrical conductivity, density, other properties, as well as what are the features of the structure of aluminum alloys and their chemical composition.
History of discovery
In the 16th century, the famous Paracelsus took the first step towards aluminum mining. From alum, he isolated “alum earth,” which contained the oxide of a then unknown metal. In the 18th century, the German chemist Andreas Marggraff returned to this experiment. He named the aluminum oxide “alumina,” which means “astringent” in Latin. At that time, the metal was not popular because it was not found in its pure form. For many years, English, Danish and German scientists tried to isolate pure aluminum. In 1855, at the Paris World Exhibition, the metal aluminum created a sensation. Only luxury items and jewelry were made from it, since the metal was quite expensive. At the end of the 19th century, a more modern and cheaper method of producing aluminum appeared. In 1911, the first batch of duralumin, named after the city, was produced in Duren. In 1919, the first airplane was created from this material.
Aluminum surface treatment
The natural metallic surface of aluminum is aesthetically attractive for many products even without additional processing. This natural protective oxide coating is clear and can be thickened by anodizing. This achieves additional surface protection without compromising the appearance of the product.
Categories
Aluminum allows the use of a large number of surface treatment methods. Types of surface treatments are divided into four broad categories:
- mechanical,
- chemical,
- electrolytic coatings and
- non-electrolytic coatings.
Some of them change its appearance, others give the surface specified properties, for example, corrosion resistance. Mechanically and chemically it is possible to create different surface textures: from rough to mirror-smooth.
Anodizing
Anodizing aluminum makes it possible to make the natural surface matte or colored. Aluminum anodizing technology includes the use of various electrolytes and electrical parameters - voltage and current (Figure 9).
Figure 9 – Principle of anodizing aluminum
Coloring
Various painting methods are widely used for aluminum: from applying “wet” paint to powder coating (Figure 10) and electrolytic coating of other metals.
Figure 10 – Vertical powder coating of aluminum profiles
Physical properties
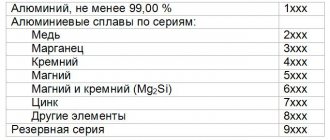
Aluminum metal is characterized by high electrical conductivity, thermal conductivity, resistance to corrosion and frost, and ductility. It lends itself well to stamping, forging, drawing, and rolling. Aluminum can be welded well with various types of welding. An important property is its low density of about 2.7 g/cm³. The melting point is about 660°C. The mechanical, physicochemical and technological properties of aluminum depend on the presence and amount of impurities that worsen the properties of the pure metal. The main natural impurities are silicon, iron, zinc, titanium and copper.
According to the degree of purification, aluminum is distinguished between high and technical purity. The practical difference is the difference in corrosion resistance to certain environments. The purer the metal, the more expensive it is. Technical aluminum is used for the production of alloys, rolled products and cable and wire products. High purity metal is used for special purposes. In terms of electrical conductivity, aluminum is second only to gold, silver and copper. And the combination of low density and high electrical conductivity allows it to compete with copper in the field of cable and wire products. Long-term annealing improves electrical conductivity, while cold hardening worsens it.
The thermal conductivity of aluminum increases with increasing purity of the metal. Impurities of manganese, magnesium and copper reduce this property. In terms of thermal conductivity, aluminum is inferior only to copper and silver. Due to this property, the metal is used in heat exchangers and cooling radiators. Aluminum has a high specific heat capacity and heat of fusion. These figures are significantly higher than those of most metals. The higher the purity of aluminum, the more it is able to reflect light from the surface. The metal is well polished and anodized.
Aluminum has a high affinity for oxygen and is covered in air with a thin, durable film of aluminum oxide. This film protects the metal from subsequent oxidation and provides its good anti-corrosion properties. Aluminum is resistant to atmospheric corrosion, sea and fresh water, and practically does not interact with organic acids, concentrated or diluted nitric acid.
Connection of copper and aluminum wires
Recently, electrical equipment of increasingly higher power has begun to be used in everyday life and industry. During Soviet times, wiring was made mainly of cheap aluminum. Unfortunately, its performance characteristics no longer meet the new requirements. Therefore, today in everyday life and in industry very often aluminum wires are replaced with copper ones. The main advantage of the latter, in addition to refractoriness, is that during the oxidation process their conductive properties do not decrease.
Nichrome
Often when modernizing electrical networks, aluminum and copper wires have to be connected. This cannot be done directly. Actually, the electrical conductivity of aluminum and copper does not differ too much. But only for these metals themselves. The oxidizing films of aluminum and copper have different properties. Because of this, the conductivity at the junction is significantly reduced. The oxidation film of aluminum has much greater resistance than that of copper. Therefore, the connection of these two types of conductors must be made exclusively through special adapters. These could be, for example, clamps containing a paste that protects metals from the appearance of oxide. This adapter option is usually used outdoors. Branch compressors are more often used indoors. Their design includes a special plate that eliminates direct contact between aluminum and copper. If such conductors are not available at home, instead of twisting the wires directly, it is recommended to use a washer and nut as an intermediate “bridge”.
Chemical properties
Aluminum is a fairly active amphoteric metal. Under normal conditions, a strong oxide film determines its durability. If the oxide film is destroyed, aluminum acts as an active reducing metal. In a finely crushed state and at high temperatures, the metal interacts with oxygen. When heated, reactions occur with sulfur, phosphorus, nitrogen, carbon, and iodine. Under normal conditions, the metal reacts with chlorine and bromine. There is no reaction with hydrogen. With metals, aluminum forms alloys containing intermetallic compounds - aluminides.
Provided that the oxide film is removed, vigorous interaction with water occurs. Reactions with dilute acids occur easily. Reactions with concentrated nitric and sulfuric acid occur when heated. Aluminum reacts easily with alkalis. Practical application in metallurgy has found the property of reducing metals from oxides and salts - aluminothermy reactions.
Recycled aluminum
The source for secondary aluminum is aluminum scrap and aluminum waste in all forms and types of products, as well as slag and other waste from aluminum foundries. Primary and secondary aluminum production are closely linked. Many aluminum alloys, wrought and cast, involve various impurities that may be present in aluminum scrap and aluminum process waste. In the last decade, the use of aluminum waste in the production of various aluminum products has been steadily increasing. An example of this is the production of aluminum sheet for the production of cans for packaging beer and soft drinks.
Figure 6 – Aluminum scrap: cans for packaging beer and drinks
Details about aluminum scrap and recycled aluminum:
Handbook of aluminum recycling /Ch. Schmitz – 2014
Aluminum recycling /M. Schlesinger - 2017
Receipt
Aluminum is in first place among metals and in third place among all elements in terms of abundance in the earth's crust. Approximately 8% of the mass of the earth's crust is this metal. Aluminum is found in the tissues of animals and plants as a trace element. In nature, it is found bound in the form of rocks and minerals. The rocky shell of the earth, which is at the base of the continents, is formed precisely by aluminosilicates and silicates.
Aluminosilicates are minerals formed as a result of volcanic processes under appropriate high temperature conditions. During the destruction of aluminosilicates of primary origin (feldspars), various secondary rocks with a higher aluminum content (alunites, kaolins, bauxites, nephelines) were formed. Aluminum is included in secondary rocks in the form of hydroxides or hydrosilicates. However, not every aluminum-containing rock can be a raw material for alumina, a product from which aluminum is produced using the electrolysis method.
Aluminum is most often obtained from bauxite. Deposits of this mineral are common in countries of the tropical and subtropical zone. In Russia, nepheline ores are also used, deposits of which are located in the Kemerovo region and on the Kola Peninsula. When extracting aluminum from nephelines, potash, soda ash, cement and fertilizers are also produced along the way.
Bauxite contains 40-60% alumina. It also contains iron oxide, titanium dioxide, and silica. The Bayer process is used to isolate pure alumina. In an autoclave, the ore is heated with caustic soda, cooled, and the “red mud” (solid sediment) is separated from the liquid. Afterwards, aluminum hydroxide is precipitated from the resulting solution and calcined to obtain pure alumina. Alumina must meet high standards for purity and particle size.
Alumina (aluminum oxide) is extracted from the mined and enriched ore. The alumina is then converted into aluminum using electrolysis. The final stage is recovery by the Hall-Heroux process. The process is as follows: during the electrolysis of an alumina solution in molten cryolite, aluminum is released. The cathode is the bottom of the electrolysis bath, and the anode is carbon bars located in cryolite. Molten aluminum is deposited under a solution of cryolite with 3-5% alumina. The process temperature rises to 950°C, which is much higher than the melting point of aluminum itself (660°C). Deep purification of aluminum is carried out by zone melting or distillation through subfluoride.
Conductive Adhesive Brands
There are several manufacturers of conductive adhesive, both foreign and domestic, that guarantee high electrical conductivity.
- Contact. Probably the most famous composition among radio amateurs. Conductive adhesive contactol has high elasticity, sufficient strength, is made on a silver basis and dries quickly, which ensures quick and convenient installation. You can buy conductive glue of this brand in any amateur radio store, however, the professionals in this field themselves speak rather poorly about it. But there are also positive reviews. Contactol
- Elekont. Conductive adhesive that will be useful to every car owner. This is an epoxy compound. Reviews about it are also not encouraging. Elekont
- Done deal. This is a foreign representative of this type of glue. Conductive adhesive done deal has increased reliability and strength, which makes it better than domestic analogues. Done Deal
- Homakoll. A fairly popular brand of conductive adhesive that has long established itself in the market. It is used by large companies as an electrically conductive adhesive for floor coverings with an antistatic effect. Homakoll
- Mastix. This company introduces an electrically conductive adhesive for repairing heated rear windows. Mastix conductive adhesive is considered one of the best in this segment. Mastix
- TPK-E. The brand is distinguished by its technical characteristics. This adhesive will function in a wide range of temperatures. From -190 to +200 °C. Used in enterprises.
Application
Aluminum is used in metallurgy as a base for alloys (duralumin, silumin) and an alloying element (alloys based on copper, iron, magnesium, nickel). Aluminum alloys are used in everyday life, in architecture and construction, in shipbuilding and automotive industry, as well as in space and aviation technology. Aluminum is used in the production of explosives. Anodized aluminum (coated with colored films of aluminum oxide) is used to make jewelry. Metal is also used in electrical engineering.
Specific electrical conductivity of some substances (table)
Specific conductivity is given at +20 °C:
| Substance | Sm/m | Substance | Sm/m | Substance | Sm/m | Substance | Sm/m | Substance | Sm/m |
| silver | 62 500 000 | molybdenum | 18 500 000 | tin | 8 330 000 | mercury | 1 040 000 | marble | 10−8 |
| copper | 59 500 000 | tungsten | 18 200 000 | cast steel | 7 690 000 | nichrome | 893 000 | glass | 10−11 |
| gold | 45 500 000 | zinc | 16 900 000 | lead | 4 810 000 | graphite | 125 000 | porcelain | 10−14 |
| aluminum | 38 000 000 | nickel | 11 500 000 | nickel silver | 3 030 000 | sea water | 3 | quartz glass | 10−16 |
| magnesium | 22 700 000 | pure iron | 10 000 000 | constantan | 2 000 000 | the ground is wet | 10−2 | amber | 10−18 |
| iridium | 21 100 000 | platinum | 9 350 000 | manganin | 2 330 000 | distilled water | 10−4 |