Which metal is the most fusible after mercury?
The most fusible of all metals known today is mercury.
The name of the person who first discovered this miracle metal was lost in the depths of millennia, but it is known for certain that mercury was familiar to humanity several centuries before our era.
Today it can no longer be said that mercury is the only metal that exists in the liquid phase under conditions that are considered normal (750.06 mm Hg, 25 ° C).
Francium, a very rare metal, is in a liquid state already at 15 - 23o C, but the study of this substance is very difficult due to its high radioactivity and short half-life.
Cesium and gallium melt at slightly higher temperatures, namely at +28.5o C and +29.8o C, respectively. Mercury breaks all records among metals in terms of melting point.
The transition of mercury from solid to liquid occurs at minus 38.89 °C!
Until the mid-18th century, it was believed that mercury could only exist in liquid and gaseous states. The science of that time did not classify mercury as a metal at all, despite the inherent properties of this class of compounds. The existence of mercury in solid form was discovered by accident. In 1734 in
Tomsk weather station observer, equestrian Cossack Salomatov, noticed that in very severe frosts the mercury in his barometer froze. He reported his observation to scientists Gmelin and Miller, members of the Academy of Sciences of St. Petersburg.
However, the unshakable belief of scientists that mercury cannot be solid prevented them from taking this information seriously.
A quarter of a century later, in the winter of 1759–1760, during scientific experiments, the freezing of mercury was discovered by the scientist Joseph Adam Brown. It was very cold in St. Petersburg that day, December 14, 1759. The thermometer readings reached -37° C. Brown set up an experiment, the purpose of which was to lower the temperature of the substance as much as possible.
The scientist mixed street snow with a small amount of nitric acid in a glass vessel and placed a mercury thermometer in this environment to measure temperature. And then Brown discovered that the mercury in the thermometer had frozen.
It was a sensational discovery! After all, until then, not a single scientific work had mentioned that mercury could exist in the solid phase.
The experience of Joseph Adam Brown was then reproduced by academicians Lomonosov, Zeiger and Epinus. They also confirmed that mercury had frozen. That same winter, in January 1760, M.V.
Lomonosov discovered that mercury in solid form, as well as in liquid form, has the property of electrical conductivity.
After Lomonosov's experiments, the controversial issue of whether mercury belongs to the class of metals was finally resolved.
Read also: Grinders with metabo speed control
low-melting metals - Color group. metals with low tm, including Zn, Cd, Hg, Sn, Pb, Bi, Ti, Sb and elements with weakening. metallic with you: Ga, Ge. [https://metaltrade.ru/abc/a.htm] Topics: metallurgy in general EN low melting metals ... Technical Translator's Guide
low-melting metals - alloys with low melting point, the main components of which are low-melting metals: Hg (tmelting = 39 °C), Ga (30 °C), In (156 °C), Sn (232 °C), Bi (271 *C) , Pb (327 °C), Cd (321 °C) and Zn (419 °C) ... Encyclopedic Dictionary of Metallurgy
Metals are simple substances that, under normal conditions, have characteristic properties: high electrical and thermal conductivity, negative temperature coefficient of electrical conductivity, the ability to reflect electromagnetic waves well, ... ... Encyclopedic Dictionary of Metallurgy
ultrapure metals - high-purity, ultra-pure metals in which the mass fraction of impurities does not exceed 1 • 10 3%. The main stages of the technology for the production of ultrapure metals: obtaining pure chemical compounds, reducing them to... ... Encyclopedic Dictionary of Metallurgy
pure metals - metals with a low content of impurities ( Encyclopedic Dictionary of Metallurgy
refractory metals - metals with tmelt > fFe = 1539 °C (for example, Cr, V, W, Mo, Nb, etc.); used as alloying additives in steel, as well as as a base for corresponding special alloys; See also: Metals alkali metals ... Encyclopedic Dictionary of Metallurgy
radioactive metals are metals that occupy places in the Periodic Table of elements with an atomic number greater than 83 (Bi), emitting radioactive particles: neutrons, protons, alpha, beta particles or gamma quanta. Found in nature: At, Ac, Np, Pa, Po... Encyclopedic Dictionary of Metallurgy
transition metals are elements Ib and VIIIb of the subgroup of the Periodic Table. In transition metal atoms, the inner shells are only partially filled. There are d metals in which there is a gradual filling of 3d (from Se to Ni), 4d (from Y to ... ... Encyclopedic Dictionary of Metallurgy
Read also: Using dinistors in power regulators
primary metals are metals obtained from ore or ore materials, as opposed to secondary metals obtained from waste and scrap (for example, primary and secondary Al); See also: Metals alkali metals pure metals ... Encyclopedic Dictionary of Metallurgy
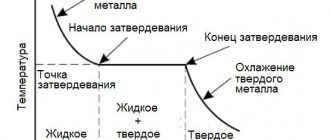
Each metal or alloy has unique properties, including its melting point. In this case, the object passes from one state to another, in a particular case, it becomes liquid from solid.
To melt it, you need to apply heat to it and heat it until the desired temperature is reached. At the moment when the desired temperature point of a given alloy is reached, it may still remain in a solid state.
As exposure continues, it begins to melt.
Mercury has the lowest melting point - it melts even at -39 °C, tungsten has the highest - 3422 °C. For alloys (steel and others) it is extremely difficult to determine the exact figure. It all depends on the ratio of the components in them. For alloys it is written as a numerical interval.
How the process works
Elements, whatever they are: gold, iron, cast iron, steel or any other, melt approximately the same. This occurs due to external or internal heating.
External heating is carried out in a thermal furnace. For internal applications, resistive heating is used, passing an electric current or induction heating in a high-frequency electromagnetic field .
The impact is approximately the same.
When heating occurs , the amplitude of thermal vibrations of molecules increases. Structural lattice defects appear , accompanied by the rupture of interatomic bonds. The period of lattice destruction and accumulation of defects is called melting.
Depending on the degree at which metals melt, they are divided into:
- low-melting - up to 600 °C: lead, zinc, tin;
- medium-melting - from 600 °C to 1600 °C: gold, copper, aluminum, cast iron, iron and most of all elements and compounds;
- refractory - from 1600 °C: chromium, tungsten, molybdenum, titanium.
Depending on what the maximum degree is, the melting apparatus is selected. It should be stronger the stronger the heating.
The second important value is the boiling degree. This is the parameter at which liquids begin to boil. As a rule, it is twice the melting point. These values are directly proportional to each other and are usually given at normal pressure.
If the pressure increases, the amount of melting also increases. If the pressure decreases, then it decreases.
Characteristics table
Metals and alloys are an indispensable basis for forging , foundry, jewelry and many other areas of production. Whatever the craftsman does ( gold jewelry , cast iron fences, steel knives or copper bracelets) , in order to work correctly, he needs to know the temperatures at which this or that element melts.
Read also: Requirements for hammers and sledgehammers
To find out this parameter, you need to refer to the table. In the table you can also find the boiling degree.
Among the most commonly used elements in everyday life, the melting point indicators are as follows:
- aluminum - 660 °C;
- copper melting point - 1083 °C;
- melting point of gold - 1063 °C;
- silver - 960 °C;
- tin - 232 °C.
Tin is often used for soldering, since the temperature of a working soldering iron is exactly 250–400 degrees; - lead - 327 °C;
- melting point of iron - 1539 °C;
- the melting point of steel (an alloy of iron and carbon) is from 1300 °C to 1500 °C.
It varies depending on the saturation of the steel with components; - melting point of cast iron (also an alloy of iron and carbon) - from 1100 °C to 1300 °C;
- mercury - -38.9 °C.
As is clear from this part of the table, the most fusible metal is mercury, which at positive temperatures is already in a liquid state.
The boiling point of all these elements is almost twice, and sometimes even higher than the melting point. For example, for gold it is 2660 °C, for aluminum - 2519 °C , for iron - 2900 °C, for copper - 2580 °C, for mercury - 356.73 °C.
For alloys such as steel, cast iron and other metals, the calculation is approximately the same and depends on the ratio of components in the alloy.
The maximum boiling point of metals is rhenium - 5596 ° C. The highest boiling point is for the most refractory materials.
There are tables that also indicate the density of metals . The lightest metal is lithium, the heaviest is osmium. Osmium has a higher density than uranium and plutonium when viewed at room temperature.
Light metals include: magnesium, aluminum, titanium. Heavy metals include most common metals: iron, copper, zinc, tin and many others. The last group is very heavy metals, these include: tungsten, gold, lead and others.
Another indicator found in the tables is the thermal conductivity of metals . Neptunium is the worst conductor of heat, and the best metal in terms of thermal conductivity is silver. Gold, steel, iron, cast iron and other elements are in the middle between these two extremes. Clear characteristics for each can be found in the required table.
Records in science and technology. Particles and substances
All known matter on Earth and beyond is composed of chemical elements. It is estimated that there are 1087 electrons in the known Universe.
The total number of naturally occurring elements is 94.
At normal temperature, 2 of them are in the liquid state, 11 are in the gaseous state and 81 (including 72 metals) are in the solid state.
The so-called “fourth state of matter” is plasma, a state in which negatively charged electrons and positively charged ions are in constant motion.
The lightest and most massive elementary particles
By April 1988, science knew of the existence of 31 stable particles, 64 meson multiplet resonances and 52 baryon multiplet resonances, which could ultimately lead to the discovery of 247 elementary particles, as well as an equal number of antiparticles.
The most massive commonly accepted particle is the 92.4 GeV neutral intermediate vector boson Z0, first discovered in May 1983.
Laboratory UA-1 of the European Organization for Nuclear Research (CERN), Geneva, Switzerland, operating at the proton-antiproton collider SPS (ultra-high energy proton synchrotron) with a beam energy of 540 GeV.
The most massive hadron is considered to be a σ-meson resonance (6S) (mass 11.02 GeV, lifetime 8.3 × 10–24 s), consisting of a beauty quark (b-quark) or a down quark (d-quark) and his antique.
It was first discovered by two groups working at the Electronic Storage Ring at Cornell University, Ithaca, New York, USA.
According to the modern theory of elementary particles, the mass of the graviton, photon and neutrino should be equal to zero.
According to estimates corresponding to various cosmological theories, the upper limits of the mass of these particles are 7.6 10–67 g for the graviton, 5.3 10–60 g for the photon and 3.2 10–32 g for the neutrino (average mass electron is 9.10939·10–28 g).
Most and least stable
From the “grand unified theory”, which describes weak, electromagnetic and strong interactions, it follows that the proton is unstable. However, according to experimental results published in 1986.
, the lifetime of a proton in the case of the most probable method of its decay (into a positron and a neutral pion) has a lower limit of 3.1·1032 years, which is more than 40 times longer than the maximum lifetime predicted by theory.
The most unstable or shortest-lived particles are the two baryon resonances N (2220) and N (2600), whose lifetime is 1.6 10–24 s, while the theoretically predicted lifetime of the intermediate vector bosons W± and Z0 is 2.6 ·10–25 s.
Newest particles
The newest particles are χ-meson resonances (2P), the discovery of which was announced in 1987 by a joint group of Columbia and New York (Stony Brook, New York, USA) universities.
The scientists used electronic storage rings from Cornell University, Ithaca, New York, USA.
Mesons consist of a b quark and its antiquark and have masses of 10.235 GeV (χb0), 10.255 GeV (χb1) and 10.269 GeV (χb2).
Most offensive substance
The most foul-smelling of the 17 thousand.
The substances registered so far in the world are, although this may be subjective, ethyl mercaptan (C2H5SH) and butyl selenomercaptan (C4H9SeH).
The smell of each of them is reminiscent of a mixture of rotting cabbage, garlic, onions, burnt toast and sewer gases.
The most expensive perfumes
The retail price of a perfume is determined more by advertising considerations than by the cost of components and packaging.
Chicago began selling in March 1984.
a cologne called Andron, containing trace levels of the attractant pheromone androstenol, priced at $97 per gram.
The most powerful poison
The disease rickettsiosis, or Q fever, can be caused by a single microorganism. However, only in one case out of a thousand does it lead to death.
About 10 organisms, Francisella tularenesis (formerly Pasteurella tularenesis), can cause the disease tularemia, variously called alkali disease, Francis disease, or “deer fly fever.” It causes death in 10 cases out of a thousand.
The most powerful nerve gas
VX gas is 300 times more toxic than phosgene (COCl2) used during the First World War. It was created at the Chemical Defense Experimental Laboratories, Porton Down, UK, in 1952.
Patent applications were filed in 1962 and published only in February 1974. They stated that the substance was ethyl S-2-diisopropylaminoethyl methyl phosphonotylate.
The lethal dose is 10 mg min/m3 in air or 0.3 mg orally.
The most powerful absorbent
August 18, 1974
The US Department of Agriculture Research Service announced the creation of a superabsorbent called “H-span”, which contains 50% starch derivative and 25% each of acrylamide and acrylic acid. After treatment with iron, the absorbent is able to absorb a mass of water 1300 times greater than its own mass.
The finest powder
The grinding limit is solid helium, which, as was established back in 1964, should be a monoatomic powder.
The most poisonous artificial substance
TCDD, or 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin, discovered in 1872, is lethal at a concentration of 3.1 × 10–9 mol/kg, which is 150 thousand times stronger than a similar dose of cyanide.
The most refractory substance
Tantalum carbide TaC0-88 melts at a temperature of 3990°C.
Substance with the lowest density
The substance with the lowest density are silicon aerogels, in which spheres of bonded silicon and oxygen atoms form long strands separated by air layers. In February 1990, at the National Laboratory.
Lawrence Livermore, California, USA, the lightest of these aerogels was obtained with a density of only 0.005 g/cm3.
This substance is supposed to be used in space research to collect micrometeorites present in the tails of comets.
Substance with the highest superconducting temperature
In March 1988, the phenomenon of superconductivity was observed at the IBM Research Center in San Jose, California, USA, at a temperature of –148°C. The conductor was a mixture of oxides of thallium, calcium, barium and copper - Тl2Са2Ва2Сu3О x
.
The sweetest substance
Talin, obtained from the husk of katemfe (Thaumatococcus Daniellii), found in West Africa, is 6150 times sweeter than a 1% sucrose solution.
The most bitter substance
The bitter taste of Vilex (denatonium benzoate) is felt when one part of it is dissolved in 100 million parts of the solution.
Previously published:
Guinness Book of Records, 1998
February 3, 2002
Electronic version:
© NiT. Articles, 1997
Source: https://nt.ru/tp/in/rnt01.htm
Low-melting metals list - masakarton.com
| H | He | |||||||||||||
| Li | Be | B | C | N | O | F | Ne | |||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | |||||||
| K | Ca | Sc | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Tc | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||
| Cs | Ba | La | * | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||
| Fr | Ra | Ac | ** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | |||
| * | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu |
| ** | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | MD | No | Lr |
| Refractory metals | Expanded group of refractory metals[1] |
See also: Refractory alloys
Refractory metals
- a class of chemical elements (metals) that have a very high melting point and resistance to wear. The expression refractory metals
Most often used in disciplines such as materials science, metallurgy and engineering sciences. The definition of refractory metals applies to each element of the group differently.
The main representatives of this class of elements are the elements of the fifth period - niobium and molybdenum; sixth period - tantalum, tungsten and rhenium. All of them have a melting point above 2000 °C, are chemically relatively inert and have an increased density.
Thanks to powder metallurgy, they can be used to produce parts for various industries.
Definition
Most definitions of the term refractory metals
define them as metals having high melting points. By this definition, metals must have a melting point above 4,000 °F
(2,200
°C
). This is necessary for their identification as refractory metals[2]. Five elements - niobium, molybdenum, tantalum, tungsten and rhenium are included in this list as the main ones [3], while a broader definition of these metals allows us to include in this list also elements with a melting point of 2123 K (1850 °C) - titanium, vanadium, chromium, zirconium, hafnium, ruthenium and osmium. Transuranium elements (which are located behind uranium, all isotopes of which are unstable and are very difficult to find on earth) will never be classified as refractory metals[4].
Physical properties
Properties of the fourth group of elements
| Name | Niobium | Molybdenum | Tantalum | Tungsten | Rhenium |
| Melting temperature | 2750 K (2477 °C) | 2896 K (2623 °C) | 3290 K (3017 °C) | 3695 K (3422 °C) | 3459 K (3186 °C) |
| Boiling temperature | 5017 K (4744 °C) | 4912 K (4639 °C) | 5731 K (5458 °C) | 5828 K (5555 °C) | 5869 K (5596 °C) |
| Density | 8.57 g cm³ | 10.28 g cm³ | 16.69 g cm³ | 19.25 g cm³ | 21.02 g cm³ |
| Young's modulus | 105 GPa | 329 GPa | 186 GPa | 411 GPa | 463 GPa |
| Vickers hardness | 1320 MPa | 1530 MPa | 873 MPa | 3430 MPa | 2450 MPa |
The melting point of these elements is the highest, excluding carbon and osmium. This property depends not only on their properties, but also on the properties of their alloys.
Metals have a cubic system, with the exception of rhenium, in which it takes the form of a hexagonal close packing.
Most of the physical properties of the elements in this group vary significantly because they are members of different groups[5][6].
Resistance to creep deformation is a defining property of refractory metals. In ordinary metals, deformation begins at the melting point of the metal, and hence creep deformation in aluminum alloys begins at 200 °C
, while for refractory metals it starts at 1500 °C
. This resistance to deformation and high melting point allows refractory metals to be used, for example, as parts of jet engines or in the forging of various materials[7][8].
Chemical properties
| This section is not completed. You will help the project by correcting and expanding it. |
In open air they undergo oxidation. This reaction slows down due to the formation of a passivated layer. Rhenium oxide is very unstable because when a dense flow of oxygen is passed through, its oxide film evaporates. All are relatively resistant to acids.[5]
Indium
As a simple substance, indium is very light, malleable and soft, so much so that it even leaves a mark if it is passed over paper. It is also one of the most fusible metals, but is only affected by temperatures above 157°C. It boils at 2072 degrees.
Like gallium, indium does not form its own deposits, but is found in various ores. Due to its dispersion in nature, the metal is quite expensive. It is used in microelectronics, for the manufacture of low-melting alloys, solders, and liquid crystal screens for equipment.
View gallery
Application
Refractory metals are used as light sources, parts, lubricants, in the nuclear industry as ARC, and as a catalyst.
Because they have high melting points, they are never used as an open-air smelting material. In powder form, the material is compacted using melting furnaces.
Refractory metals can be processed into wire, ingot, rebar, tin or foil.
Tungsten and its alloys
Main article: Tungsten
Tungsten was discovered in 1781 by the Swedish chemist Carl Wilhelm Scheele. Tungsten has the highest melting point of all metals - 3422 °C
(6170 °F
)
Tungsten.
Rhenium is used in alloys with tungsten in concentrations up to 22 %
, which improves refractoriness and corrosion resistance. Thorium is used as an alloying component of tungsten. This increases the wear resistance of materials.
In powder metallurgy, components can be used for sintering and subsequent application. To obtain heavy tungsten alloys, nickel and iron or nickel and copper are used. tungsten in these alloys usually does not exceed 90%.
The mixing of the alloying material with it is low even during sintering[9].
The most refractory metal on earth
Curious people are probably interested in the question, which metal is the most refractory? Before answering it, it is worth understanding the concept of refractoriness itself.
All metals known to science have different melting points due to varying degrees of stability of bonds between atoms in the crystal lattice.
The weaker the bond, the lower the temperature required to break it.
The world's most refractory metals are used in their pure form or in alloys to produce parts that operate under extreme thermal conditions.
They can effectively withstand high temperatures and significantly extend the operating life of the units.
But the resistance of metals of this group to thermal effects forces metallurgists to resort to non-standard methods of their production.
Which metal is the most refractory?
The most refractory metal on Earth was discovered in 1781 by the Swedish scientist Carl Wilhelm Scheele. The new material is called tungsten. Scheele was able to synthesize tungsten trioxide by dissolving the ore in nitric acid.
The pure metal was isolated two years later by Spanish chemists Fausto Fermin and Juan José de Eluar. The new element did not immediately gain recognition and was adopted by industrialists.
The fact is that the technology of that time did not allow processing such a refractory substance, so most contemporaries did not attach much importance to the scientific discovery.
Tungsten was appreciated much later. Today, its alloys are used in the production of heat-resistant parts for various industries. The filament in gas-discharge household lamps is also made of tungsten.
It is also used in the aerospace industry for the production of rocket nozzles, and is used as reusable electrodes in gas arc welding.
In addition to being refractory, tungsten also has a high density, which makes it suitable for making high-quality golf clubs.
Tungsten compounds with non-metals are also widely used in industry.
So sulfide is used as a heat-resistant lubricant that can withstand temperatures up to 500 degrees Celsius, carbide is used to make cutters, abrasive discs and drills that can handle the hardest substances and withstand high heating temperatures. Let us finally consider the industrial production of tungsten. The most refractory metal has a melting point of 3422 degrees Celsius.
How is tungsten obtained?
Pure tungsten does not occur in nature. It is part of rocks in the form of trioxide, as well as wolframites of iron, manganese and calcium, less often copper or lead. According to scientists, the tungsten content in the earth's crust averages 1.3 grams per ton.
This is a rather rare element compared to other types of metals. tungsten in ore after mining usually does not exceed 2%.
Therefore, the extracted raw materials are sent to processing plants, where the mass fraction of metal is brought to 55-60% using magnetic or electrostatic separation.
The process of its production is divided into technological stages. In the first stage, pure trioxide is isolated from the mined ore. For this purpose, the thermal decomposition method is used.
At temperatures from 500 to 800 degrees Celsius, all excess elements melt, and refractory tungsten in the form of oxide can be easily collected from the melt.
The output is raw material with a hexavalent tungsten oxide content of 99%.
The resulting compound is thoroughly crushed and a reduction reaction is carried out in the presence of hydrogen at a temperature of 700 degrees Celsius. This allows you to isolate pure metal in powder form.
Next, it is pressed under high pressure and sintered in a hydrogen environment at temperatures of 1200-1300 degrees Celsius.
After this, the resulting mass is sent to an electric melting furnace, where, under the influence of current, it is heated to a temperature of over 3000 degrees. This is how tungsten turns into a molten state.
For final purification from impurities and obtaining a single-crystal structural lattice, the zone melting method is used.
It implies that at a certain point in time only a certain zone of the total area of the metal is molten.
Gradually moving, this zone redistributes impurities, as a result of which they ultimately accumulate in one place and can be easily removed from the alloy structure.
Finished tungsten arrives at the warehouse in the form of bars or ingots, intended for the subsequent production of the desired products. To obtain tungsten alloys, all constituent elements are crushed and mixed in powder form in the required proportions. Next, sintering and melting are carried out in an electric furnace.
| The main component of an aluminum alloy is, as the name implies, aluminum. Other, most common elements that are part of aluminum-based alloys include copper, iron, zinc... |
The most fusible metals: properties, features, physical characteristics
Melting point is an important characteristic that is most often applied specifically to metals. It depends on many physical properties of substances - their purity and crystal structure. Which metal is the most fusible: Li, Al, Hg, Cu? Let's find out which of them can really be called such.
The most fusible metals
Melting is the process of transition from a solid to a liquid state. It occurs under the influence of heat, but also depends on a number of physical factors, such as pressure. An important role in how easily and hard a substance can be melted is also played by its composition, the size of the crystals in the lattice, and the strength of the bonds between the atoms.
The melting point of metals varies greatly and can even have sub-zero values. It ranges from -39 to +3410 degrees Celsius. Molybdenum, tungsten, chromium, and titanium are the hardest to transform into liquid. For this process they need to be heated to a temperature of at least 2000 degrees.
The most fusible metals are gallium, mercury, lithium, tin, lead, zinc, indium, bismuth, and thallium. Read more about some of them below.
Mercury
Useful in many areas, but poisonous metal was known even before our era. Mercury was used by ancient and medieval physicians to treat venereal and many other diseases; alchemists tried to make gold from it. Today it is used in electrical engineering, instrument making and organic chemistry.
Ruth is the most fusible metal on the planet. Under normal room conditions, it is always liquid, since its melting point is -39 degrees. Its vapors are very dangerous, so mercury is contained only in containers and special glass flasks. It acts like a poison on the body, poisoning it and disabling the nervous, immune, respiratory and digestive systems.
Gallium
The second most fusible metal is gallium. It becomes liquid at temperatures above 29.5 degrees Celsius, and can be softened simply by holding it in your hands for a while. Under normal conditions, gallium is very brittle, easily amenable to mechanical action, and has a light silver, somewhat bluish tint.
The metal is very dispersed in the earth's crust and is not found in the form of nuggets. In nature, it is found in various minerals, such as garnet, muscovite, tourmaline, chlorite, and feldspar. In addition, it is found in sea water. Gallium is used in high-frequency electronics, for the manufacture of mirrors and various alloys.
Tin
Tin melts at temperatures above 231 degrees Celsius. It is a ductile and soft metal, light silver in color. It exists in four allotropic modifications, two of which appear only at high pressure.
Tin is fairly dispersed in nature, but can form its own minerals, such as stannine and cassiterite. It is used as a coating for metals to enhance their resistance to corrosion, as well as for the production of tin, foil, various alloys, tableware and parts for musical instruments.
Lithium
Lithium is the most fusible metal, becoming a liquid at a temperature of 180 degrees. It is soft, easily forged and machined. It belongs to the alkali metals, but is much less active than other members of the group. It reacts slowly with moist air, and remains practically stable in a dry atmosphere
The metal is found in spodumene, lepidolite, in deposits with tin, bismuth and tungsten, found in sea water and in stellar space objects. Lithium is often used for the manufacture of galvanic cells, batteries, as an oxidizer, and also in pyrotechnics. In alloys with cadmium, copper and aluminum it is used in space, military and aviation technology.