When welding, the parts are exposed to high temperatures, so it is very important to attach importance to the melting temperature of metals, taking it into account during the work process, since these indicators play an important role in the current parameters. In the burner, when gas is burned during the action of an electric arc, thermal power is created in order to destroy the crystal lattice of the metal. The melting characteristics of metals are given attention when selecting materials for the construction of units subject to frictional forces or metal structures that are exposed to temperatures.
What is melting point?
To find out at what temperature the metal melts, in laboratory conditions, the starting point at the beginning of the melting process is calculated to the hundredth of a degree. Moreover, this indicator does not depend on the force applied to the part.
When a certain pressure is created under vacuum conditions, metal blanks have the same melting point. This phenomenon can be explained by the accumulation of energy inside the substance, during which the bonds between molecules are destroyed.
Tungsten
Tungsten metal has the highest melting point. Only the nonmetal carbon ranks higher in this indicator. Tungsten is a light gray shiny substance, very dense and heavy. It boils at 5555 °C, which is almost equal to the temperature of the Sun's photosphere.
At room conditions, it reacts weakly with oxygen and does not corrode. Despite its refractoriness, it is quite ductile and can be forged even when heated to 1600 °C. These properties of tungsten are used for incandescent filaments in lamps and picture tubes and electrodes for welding. Most of the mined metal is alloyed with steel to increase its strength and hardness.
Tungsten is widely used in the military sphere and technology. It is indispensable for the manufacture of ammunition, armor, engines and the most important parts of military vehicles and aircraft. It is also used to make surgical instruments and boxes for storing radioactive substances.
Difference between melting and boiling points
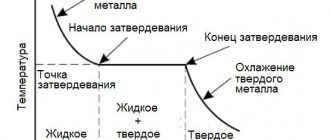
The melting point of metals is the point at which a solid crystalline substance transitions into a liquid state. In the composition of the melt, the molecules do not have their own location; they are held due to the force of attraction, therefore, in a liquefied state, the volume is retained, but the shape is lost.
During the boiling process, a loss of molecular volume occurs, and the molecules interact sluggishly with each other, moving chaotically in different directions, lagging behind the surface. Boiling point is the process by which the pressure level of metal vapor is balanced with the pressure of the external environment.
Mercury
Mercury is the only metal whose melting point is minus. In addition, it is one of two chemical elements whose simple substances, under normal conditions, exist in the form of liquids. Interestingly, the metal boils when heated to 356.73 °C, and this is much higher than its melting point.
It has a silvery-white color and a pronounced shine. It evaporates already at room conditions, condensing into small balls. The metal is very toxic. It can accumulate in human internal organs, causing diseases of the brain, spleen, kidneys and liver.
Mercury is one of the seven first metals that man learned about. In the Middle Ages it was considered the main alchemical element. Despite its toxicity, it was once used in medicine as part of dental fillings, and also as a cure for syphilis. Now mercury has been almost completely eliminated from medical preparations, but it is widely used in measuring instruments (barometers, pressure gauges), for the manufacture of lamps, switches, and doorbells.
Melting point of various metals
According to knowledge from the section of physics, the process of transforming a solid into a liquid occurs only in bodies with a crystal lattice. The melting point of metals and alloys occurs in a different range of values. But it is very problematic to accurately calculate the boundary temperature of phase states in alloys. For pure elements, every degree is significant if these are compounds with slight fusibility.
Iron
The melting point of iron compounds must be high. If the element is technically pure, it melts at a temperature of 1,539 °C. Its substance contains sulfur inclusions, so its extraction requires a liquid state. Purified iron is also obtained through the electrolysis of metal salts.
Cast iron
Cast iron is considered the best material for melting. It has good fluid flow and shrinkage properties, so it can be used effectively in the casting process. Below are the boiling temperature indicators for cast iron:
A gray type of cast iron whose temperature reaches 1,260 °C. And when poured into molds, it increases to 1,400 °C.
A white variety of cast iron whose temperature rises to 1,350 °C.
One of the important points is that the temperature of cast iron is 400 units less than the same steel. Therefore, the processing of this material is less energy intensive.
Steel, melting point
The average melting point of steel is 1400 °C.
Steel is an iron-containing alloy containing carbon. Its main characteristic is strength. This is achieved due to the fact that it retains the parameters of volume and shape for a long time. In this case, the arrangement of molecules in the substance is in a balanced state. That is why a balance is achieved between the force of attraction and the force of repulsion.
The melting range of steel is higher than that of cast iron, so it is more energy-consuming.
Stainless steel
The melting point of stainless steel falls in the middle range between cast iron and steel. Stainless steel is a substance made of alloy steel that has anti-corrosion properties due to the chromium content of 11% or more in its composition.
The melting point of stainless steel ranges from 1,300 to 15,000 °C.
Aluminum and copper
The melting point of aluminum is 6,600 °C, so it has established itself as one of the medium-melting metals. Melting of pure copper compounds occurs at a temperature of 10,830 °C, and of alloys - 930 - 11,400 °C.
Silver and gold
Silver in its pure form melts at a temperature of 9,620 °C. Moreover, at the melting point of silver, it can be compared with the melting point in degrees of copper alloys.
Gold melts at a temperature of 10,640 °C.
Mercury
Mercury has the lowest melting point with a negative value. It is - 38.80 °C.
Concept of temperature scale
Some non-metallic objects also have similar properties. The most common is water. A temperature scale was developed regarding the properties of the liquid that occupies a dominant position on Earth. The reference points are the temperature of changes in the aggregative states of water:
- Transformations from liquid to solid and vice versa are taken to be zero degrees.
- Boiling (vapor formation inside a liquid) at normal atmospheric pressure (760 mm Hg) is taken to be 100 ⁰C.
In addition to the Celsius scale, in practice temperature is measured in degrees Fahrenheit and on the absolute Kelvin scale. But when studying the properties of metal objects, other scales are used quite rarely.
Melting point table
| Low-melting metals | |
| Lithium | + 180 °C |
| Potassium | + 63.60 °C |
| Indium | + 156.60 °C |
| Tin | + 2 320 °C |
| Thallium | + 3,040 °C |
| Cadmium | + 3 210 °C |
| Lead | + 3 270 °C |
| Zinc | + 4 200 °C |
| Medium melting metals | |
| Magnesium | + 6 500 °C |
| Aluminum | + 6 600 °C |
| Barium | + 7 270 °C |
| Silver | + 9 600 °C |
| Gold | +10 630 °C |
| Manganese | + 12 460 °C |
| Copper | + 10 830 °C |
| Nickel | + 14 550 °C |
| Cobalt | + 14 950 °C |
| Iron | + 15 390 °C |
| Duralei | + 6 500 °C |
| Brass | + 950 – 10 500 °C |
| Cast iron | + 1,100 – 13,000 °C |
| Refractory metals | |
| Titanium | + 16 800 °C |
| Platinum | + 17 690 °C |
| Chromium | + 19 070 °C |
| Zirconium | + 18 550 °C |
| Vanadium | + 19 100 °C |
| Iridium | + 24 470 °C |
| Molybdenum | + 26 230 °C |
| Tantalum | + 30 170 °C |
| Tungsten | + 34 200 °C |
At what temperature does it melt?
Metal elements, whatever they are, melt almost one to one. This process occurs when heated. It can be both external and internal. The first takes place in a furnace, and for the second, resistive heating is used, passing electricity or induction heating. The impact is almost the same. When heated, the amplitude of molecular vibrations increases. Structural lattice defects are formed, which are accompanied by the breaking of interatomic bonds. The process of lattice destruction and the accumulation of such defects means melting.
What does the melting temperature depend on?
Different materials also have different melting points, at which a radical restructuring of the lattice occurs to the state of liquid. Metal products and alloy products have the following features:
- Different materials also have different melting points, at which a radical restructuring of the lattice occurs to the state of liquid. Metal products and alloy products have the following features:
- They are rarely found in their natural form, i.e. without impurities. It is the composition that determines what the melting temperature should be. An example is tin, to which silver inclusions are added. Thanks to impurities, the material begins to become resistant to temperature.
- There are alloys that, due to their chemical composition, transform into a liquid state when the thermometer rises just above + 1,500 °C. There are also alloys that “stick” if they are heated to 30,000 °C.
- It is worth considering the fact that one of the most important properties of substances is their melting point. An example is aviation technology.
Electrical conductivity:
All metals conduct electricity , due to the presence in their crystal lattices of mobile electrons that move under the influence of an electric field.
Silver, copper and aluminum have the highest electrical conductivity. For this reason, the last two metals are most often used as wire materials. Sodium also has very high electrical conductivity. In experimental equipment, attempts are known to use sodium conductors in the form of thin-walled stainless steel pipes filled with sodium. Due to the low specific gravity of sodium, with equal resistance, sodium “wires” are much lighter than copper and even somewhat lighter than aluminum.
Types of metal alloys
The types of metal alloys vary based on their melting point, so the alloy variants are as follows:
- Low-melting (tin, zinc, lead, bismuth) with a melting point of no more than 600 °C.
- Medium melting (aluminum, magnesium, nickel, iron) with a temperature of 600 - 1,600 °C.
- Refractory (molybdenum, tungsten, titanium) with a temperature of more than 1,600 °C.
Next, we’ll tell you a little about the types of steels, wood alloy and solders.
Features of Carbon Steel
This material contains an admixture of carbon, approximately 2.13%. Moreover, it is devoid of alloying additives, but contains impurities of silicon, manganese and magnesium.
Features of Alloy Steel
In addition to the carbon and iron content, additional elements are added to it to improve its properties.
Features of stainless steel
Stainless steel differs from carbon steel due to the content of the element chromium in its composition, due to the properties of which it is not susceptible to oxidation, and, therefore, to rust.
Features of tool steel
It also has a carbon composition (0.8 - 0.9%). Demonstrates hardness, strength, and is easy to process. Used in the manufacture of instruments, such as medical ones.
Wood's alloy
It is a material used in soldering parts for radio receivers, as well as in electroplating, when working in laboratory conditions with pesticides.
Soldering alloys
Another name for them is solders. Materials for solders are different. It all depends on what is included in the materials that need to be combined. For example, aluminum requires one alloy of solder, but copper is completely different.
Plastic:
Most metals are ductile, meaning metal wire can be bent without breaking. This occurs due to the displacement of layers of metal atoms without breaking the bond between them.
The most ductile are gold, silver and copper. Gold can be used to make foil 0.003 mm thick, which is used for gilding products. However, not all metals are ductile. Wire made of zinc or tin crunches when bent; When deformed, manganese and bismuth hardly bend at all, but immediately break.
Plasticity also depends on the purity of the metal. Thus, very pure chromium is very ductile, but, contaminated with even minor impurities, it becomes brittle and harder. Some metals, such as gold, silver, lead, aluminum, osmium, can grow together, but this can take decades.