Story
One of the most famous places where bronze items were found was located in the Kuban River area. In this place, archaeologist Nikolai Veselovsky in 1897 excavated the so-called Maykop culture, which existed in the second half of the 4th millennium BC.
Bronze artifacts found in the Maikop burial mounds were made mainly from an alloy of copper and arsenic, so it is believed that historically these alloys, called arsenic bronzes, .
It was in no way inferior in its properties to alloys of copper with tin or lead, and even surpassed them in a number of characteristics . It was widely used in various areas of human activity of those times, from the manufacture of critical parts to jewelry.
Bronze composition
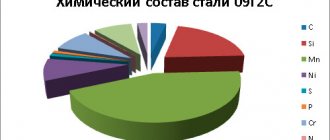
Bronze is an alloy of copper with metals such as tin, aluminum, lead, beryllium, and non-metals arsenic, silicon and phosphorus. In addition, such alloys can be additionally alloyed with phosphorus, zinc, manganese, iron and nickel.
The composition of bronze depends on the grade of the alloy and is indicated in its designation. For example, the composition of an alloy labeled BrAMts7−1 includes 7% aluminum, 1% manganese and 92% copper .
Thus, the main component of this metal is copper (from 35% to 90% and above). The second component can be either arsenic, or tin or beryllium, lead, aluminum, silicon and other components. To impart special properties, additional components can be added - zinc, iron, nickel, manganese, phosphorus and others.
BronzeMania
The main component of bronze is copper, to which other metals (usually tin) are added. At the same time, the share of other substances is no more than 2.5%, which makes it possible to improve the performance of the resulting alloy.
If copper is combined with zinc, brass is obtained; when zinc is replaced with nickel, a cupronickel composition is obtained. There are other options. For example, BrA5 is a type of bronze obtained by adding aluminum.
We work with the BrO5 brand, made on the basis of tin, since this material fully complies with state requirements.
A little about history
The first bronze products appeared in the 3rd century BC.
The Middle East is considered the birthplace of this amazing metal - the oldest finds of a compound of copper and tin were found in Iran, as well as Syria, Turkey and Iraq.
Most often, household and work items were made from bronze. The most common surviving items are household, military and jewelry items.
Then came a period when this metal became the main source of the monetary industry - coins of various denominations were made from it. Around the 5th century AD. The production of bronze sculptures began in Hellas. This is where the tradition of making bronze miniatures and figurines originates, which is still relevant today.
With the onset of the Middle Ages, bronze became part of weapons and became the main resource for casting cannons, cannonballs, and shells. Bell casters also paid attention to this metal - bronze makes beautiful products that give a deep and pleasant sound.
Bronze spear tip (7th-4th century BC).
How do the species differ?
Alloys are classified depending on the selected components. Bronze made with the addition of tin often also contains lead or phosphorus - this provides an alloying effect.
Due to tin, the alloy becomes harder and more durable, withstands melting better and retains its shape well.
The resulting material is easy to grind, and the presence of special components allows you to achieve higher performance and visual performance.
There is also bronze, which does not contain tin. Such options have a new structure that differs from the traditional one, but in terms of their properties they are almost equal to the classic alloy.
The technical properties of a metal can affect its performance.
Casting material is formed by the method of making decorative and stylish products (for example, our products). It is also widely used in the production of bearings, parts of complex mechanisms, as well as components for devices designed to operate in sea water.
Deformable materials are designed to be formed mechanically. In this case, the metal is cut, forged, and covered with corrugation. As a rule, this option is flexible and relatively soft - cables, tapes, rods and sheet products are produced from it.
Bronze rod.
Properties of bronze
When considering this alloy in comparison with other metal mixtures (for example, zinc composition), it is worth noting that real bronze is impervious to natural processes of destruction, lasts a long time and is resistant to aggressive influences (vibration, friction). It also remains durable and beautiful even with prolonged contact with water, air, acidic environments or salt solutions. Most types of bronze can be soldered or welded.
The color of the alloy depends on the components included in its composition. The lightest type is white. Dark classes have a reddish tint.
The following additives affect the tone and quality of bronze:
- zinc and lead reduce susceptibility to friction;
- aluminum and silicon extend service life, protect against corrosion and deformation;
- nickel and iron increase the ability of the alloy to recrystallize, making the substance smooth and homogeneous;
- silicon or manganese is added to increase resistance to rust, oxide deposits and intense heat;
- a material not intended to conduct electricity is made with the addition of chromium or beryllium.
The most popular classes of bronze alloy used in industry are:
- Beryllium (due to hardness). Can be hardened and is elastic. With the natural or artificial aging of a metal, its increased resistance to mechanical processes is manifested. This indicator is often enhanced by preliminary deformation. Serves for the manufacture of large and small machine parts, as well as for the production of equipment.
- Aluminum (due to high density). It is resistant to chemicals, does not change under the influence of natural factors, and is suitable for use in sea water. Easy to process and cut, popular in the production of flat and strip products.
- Silicon-zinc (advantage: excellent fluidity). Does not create sparks during machining (turning, milling). Suitable for casting complex or decorative shapes.
- Lead – resistant to friction and shock. Due to these indicators, it is more often used for parts that carry a large load.
- Tin - combines all the above advantages, and therefore is in greatest demand.
How to get bronze
Bronze making is a demanding and rather difficult process in which auxiliary metals are introduced into molten copper. Smelting is carried out in forges or induction furnaces. For heating, natural fuel (coal) or flux is used.
The first stage is placing copper in the furnace and heating it until it reaches a liquid state. After this, phosphorous copper is introduced into the substance, to which alloying components are later added. The resulting alloy is mixed, and a new processing temperature is set. At the final stage, copper phosphorous is again used, which allows you to get rid of any oxidation.
Melting bronze is simple, and therefore this metal is often used for casting art objects and miniatures. Using special forms and filling them out correctly, the specialists of the Bronzamania workshop produce products of ideal appearance. Blanks intended for artistic casting are made in round or flattened format.
Bronze in a molten state.
Application of bronze
Impeccable performance qualities have made bronze one of the most common materials in mechanical engineering, aviation, shipbuilding and large industry.
This metal is not susceptible to moisture, does not wear out, and is almost impossible to deform.
Therefore, bronze is used in the production of rolled products intended for use in aggressive chemical environments, as well as for the production of parts and pipes of various profiles.
Reliability and long service life are additional characteristics due to which bronze has become widely known in the field of sculpture and art. Interior parts are made from it - candlesticks, chandelier bodies, decor.
Therefore, the specialists of the Bronzamania workshop can guarantee the long service life of all products available for sale - our products retain their beautiful appearance and functionality for decades, without reacting to weather conditions and other adverse factors.
Ready product.
Features of bronze and properties
The main properties of all bronze alloys are ductility and hardness. Depending on the ratio of the main and additional components, a wide variety of new properties can be obtained. In addition, the amount of copper in the alloy determines its color.
So, golden bronze will be obtained if the alloy contains about 85% copper, and if its amount is reduced to 50%, an alloy will be obtained that has a silvery color. Reducing the amount of copper to 35% and below will lead to the output of gray and even black bronze, and increasing the amount of copper to 90% and above will lead to the formation of red bronze.
One of the old brands of bronze alloys is bell bronze , which is still used today for casting bells. It contains 20% tin and 80% copper. Its disadvantage is increased fragility due to the high tin content in the alloy.
As mentioned above, the most commonly used are alloys of copper and tin with the addition of a small amount of other components. The widespread use of such alloys is due, first of all, to historical reasons that led to the displacement of arsenic bronze from production.
Such reasons are the following:
- the development over many centuries of deposits of tennantite and other faded ores rich in copper and arsenic. Such ores were most convenient for the production of arsenic bronze, since they did not lie very deep, which made the production process cheaper compared to other sources of copper and arsenic;
- the high toxicity of the production of such bronze, caused by the presence of arsenic in the deposits, which inevitably led to loss of health and further ability to work for experienced metallurgists and blacksmiths;
- unsuitability of metallurgical defects and broken products made of arsenic bronze for further remelting into high-quality metal. At best, such products were used to make jewelry or non-essential parts.
The alloys of copper and tin that replaced arsenic bronzes, although they were more expensive to produce, were economically preferable , since the development of horse-drawn transport and the resulting establishment of trade relations between cities and countries led to an increase in the import of non-arsenic bronze.
Types of bronze and characteristics
The development of large-scale industrial production generally led to the fact that tin bronzes became almost the most widespread type of bronze. And only in the last hundred years this type began to be replaced by copper alloys with tin substitutes, such as aluminum, silicon and, especially, beryllium bronzes.
Thus, the following types exist:
- tin-free. It includes bronze, in which the second components are aluminum, silicon, beryllium and other metals and non-metals. Each of these components gives it special properties. For example, aluminum gives the alloy increased anti-friction properties and high corrosion resistance, beryllium increases strength and hardness, and silicon and zinc improve its fluidity and abrasion resistance;
- tin. A copper-tin alloy in which copper predominates. It is one of the first to be mastered by man. It has high hardness and strength compared to pure copper, and is also more fusible. In such alloys, tin is always the second most abundant after copper and the main alloying component.
The third in quantity are additional components such as arsenic, zinc and lead . Due to its very low shrinkage, this metal is mainly intended for casting, as it is difficult to work with pressure, cutting and sharpening. Even the tendency to segregation and low fluidity do not prevent the use of this alloy for the manufacture of configurationally complex castings, including in artistic casting.
Bronze with the addition of zinc is called “Admiralty ” and is used for the manufacture of parts that have frequent or constant contact with sea water (shipbuilding). This feature is due to the fact that zinc gives the alloy increased corrosion resistance in the specified environment.
However, to make bronze resistant to corrosion in salty seawater, it is increasingly being enriched with aluminum and nickel . Such alloys, often called “marine”, are used to manufacture elements of oil platforms operating on sea and ocean shelves.
To give bronze additional characteristics, of phosphorus, silver, zinc, arsenic, manganese and other components are alloyed into it Thus, adding a small amount of silver increases the electrical conductivity of bronze and makes it comparable to the electrical conductivity of copper.
Aluminum bronze is a base alloy - Metalist's Guide
Bronze is a double or multi-component alloy consisting of copper and other elements that improve the basic properties of the metal, except zinc. Such elements are called alloying elements. Bronze contains more than 2.5% by weight.
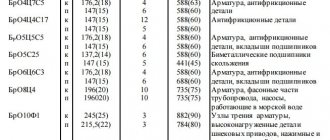
Manganese, tin, beryllium, lead, silicon, chromium, phosphorus, iron, aluminum and other elements are used as alloying components. Alloys are marked with the combination “Br”, letters that indicate the main alloying components and numbers indicating their content. For example: BrO5 - tin bronze, BrA5 - aluminum bronze.
The chemical composition of bronze alloys and their grades are determined by the corresponding GOSTs. You can buy bronze on our website.
Bronze, based on copper and tin, is one of the oldest alloys produced by man. In the 3rd millennium BC, bronze products appeared in Mesopotamia and Southern Iran.
Everything necessary for human life was made from this alloy in ancient times.
Archaeologists discovered weapons (daggers, axes, arrowheads, swords), furniture and interior items (mirrors), as well as dishes (jugs, vases, plates).
In addition, coins and all kinds of jewelry were made from bronze. Around the 5th-4th centuries BC, ancient Greek sculptors learned to cast large bronze statues; by the way, this technology is still relevant today. In the Middle Ages, bronze was used to make cannons and artillery shells. Bells have long been cast from this alloy.
By changing the composition and size of the casting, craftsmen created bells with an amazing sound.
Bronze classification
According to their chemical composition they are distinguished:
- Tin bronzes are alloys with the main alloying component tin. In addition to tin, lead, phosphorus and zinc may be present as additional components. With the addition of tin, copper acquires greater fusibility, elasticity, and hardness. Consequently, the alloy is more amenable to polishing. Additional components improve mechanical, casting, and anti-friction properties.
- Tin-free (special) bronzes are alloys that do not contain tin as an alloying element. They are not inferior in properties to tin bronzes, and in some they even surpass them.
According to technological characteristics, bronzes are divided into:
- Deformable – well amenable to mechanical processing: stamping, corrugation, forging. their tin content is no more than 6%, which ensures the necessary ductility. Sheets, bronze wire, bronze rod, and bronze tape are made from deformable tin bronzes.
- Foundry - intended for shaped castings. Various parts for machines operating in salt sea water, bearing shells, and gears are made from cast tin bronzes.
Properties of bronze
If we compare it with brass, bronze is characterized by higher corrosion resistance, strength and anti-friction properties. It is quite stable in air, salt water, carbon dioxide solutions and solutions of many organic acids. Most types of bronze can be welded and soldered with hard and soft solders.
Depending on the amount of additives, the color of bronze can be from red to white. Let's look at how alloying elements affect the properties of bronze. Tin, nickel, silicon and aluminum increase the strength, corrosion resistance, and elastic properties of bronzes.
In combination with lead, zinc and phosphorus, anti-friction properties also increase. Nickel and iron significantly refine the grain and increase the recrystallization temperature. Silicon and manganese increase heat resistance.
Chromium, zirconium and beryllium increase the heat resistance of alloys and slightly reduce electrical conductivity.
Let's take a quick look at the most commonly used types of bronze.
- Beryllium bronze is the leader in hardness among other copper alloys. In the hardened state it has good ductility and manufacturability, and in the aged state it has high mechanical properties. The level of mechanical properties can be further increased by plastic deformation before aging. Beryllium bronze is used to make springs, membranes and tools.
- Aluminum bronze is characterized by high density, resistance to aggressive environmental factors and chemical elements, and good resistance to sea water. This type of bronze can be processed with cutting tools. Tapes and strips of pipes are made from it.
- Silica-zinc bronze makes it possible to produce products of complex shapes due to its increased fluidity in the molten state. Such bronze has a high degree of compression resistance and does not spark under mechanical stress.
- Lead bronze has excellent anti-friction properties, resists shock loads well, and is also characterized by high strength and refractoriness. It is used for heavily loaded bearings.
- Tin bronze has all the above properties and is the most widely used in modern industry.
Receipt
Bronze is produced by fusing copper and alloying components. The process takes place in electric induction furnaces or crucible furnaces. The smelting charge can consist of fresh metals, as well as production waste and secondary metals. Melting is carried out under a layer of flux or charcoal.
The required amount of coal or flux is placed in a heated furnace, and then copper is loaded. After melting and heating the copper to the appropriate temperature, the melt is deoxidized with copper phosphate.
Next, heated alloying elements are introduced into the melt. Refractory alloying elements are introduced in the form of alloys. The melt is stirred until the components dissolve and heated to the required temperature.
Before casting, the melt is again deoxidized with phosphorous copper to eliminate its oxides.
Bronze melts well and fills ingot molds evenly. The alloys are produced in the form of flat and round ingots. Ingots are processed by rolling or pressing.
The result is a wide range of rolled metal:
Application of bronze
Bronze is used in modern mechanical engineering, rocketry, aviation, shipbuilding and other industries.
Due to their resistance to mechanical abrasion and high corrosion resistance, bronze products are used for the manufacture of machine parts and devices involved in moving units in the friction process. Bronze parts require periodic replacement, that is, they are consumable.
Tin-free bronze alloys are used to make rolled products for components of chemical devices, control valves for heating systems and pipelines for other purposes.
Bronze is used for casting sculptures and monuments, as the material is durable, not exposed to atmospheric influences and is resistant to mechanical damage. Products of highly artistic forms in theaters, palaces, and halls (chandeliers, floor lamps, candelabra) are also made of bronze.
Bronze is an alloy. Characteristics of bronze
Bronze is an alloy based on copper. Auxiliary metals can be nickel, zinc, tin, aluminum and others. In this article we will look at the types, technological characteristics, chemicals. composition of bronze, as well as methods of its production.
Classification
1. Based on its chemical composition, this metal is usually divided into two groups. The first is tin bronzes. In them, tin is the main alloying element. The second is tin-free. Below we will talk about this in more detail.
2. Based on technological characteristics, bronzes are usually divided into wrought and cast bronzes. The former are well processed under pressure. The latter are used for shaped castings.
Compared to brass, this metal has much better anti-friction and mechanical properties, as well as corrosion resistance.
In fact, bronze is an alloy of copper and tin (as the main auxiliary element).
Nickel and zinc are not the main alloying elements here; for this purpose, components such as aluminum, tin, manganese, silicon, lead, iron, beryllium, chromium, phosphorus, magnesium, zirconium and others are used.
Let's figure out what this metal is. Tin bronze (the photo below shows cast parts) is an alloy whose fluidity is lower than other types. However, it has insignificant volumetric shrinkage, which makes it possible to obtain shaped bronze castings. These properties determine the active use of bronze in the casting of antifriction parts.
The alloy in question is also used in the manufacture of fittings intended for use in an aquatic environment (including sea water) or in water vapor, in oils and under high pressure. There are also so-called non-standard casting bronzes for critical purposes. They are used in the production of bearings, gears, bushings, pump parts, and low-seal rings.
Such parts are designed to work under high pressure conditions, at high speeds and low loads.
This subtype of cast tin alloys is used in the manufacture of bearings, oil seals and shaped castings.
Such bronzes are characterized by low mechanical properties, as a result of which, in the process of manufacturing bearings and bushings, they are simply applied to a steel base in the form of a very thin layer.
Alloys with a higher tin content have higher mechanical properties. Therefore they can be used without a steel base.
Tin bronzes: deformable
Alloys processed by pressure are usually divided into the following groups: tin-phosphorus, tin-zinc and tin-zinc-lead. They have found their application in the pulp and paper industry (they are used to make meshes) and mechanical engineering (the production of springs, bearings and machine parts).
In addition, these materials are used in the manufacture of bimetallic products, rods, strips, strips, gears, gears, bushings and gaskets of highly loaded machines, instrument tubes, and pressure springs.
In electrical engineering, the widespread use of bronze (deformable) is explained by its excellent mechanical properties (along with high electrical characteristics).
It is used in the manufacture of current-carrying springs, plug connectors, and contacts.
In the chemical industry, tin bronzes are used to produce spring wire, in precision mechanics - fittings, in the paper industry - scrapers, in the automotive and autotractor industries - bushings and bearings.
These alloys can be supplied in extra-hard, hard, semi-hard and soft (annealed) states. Tin bronzes are usually processed cold (by rolling or drawing). Hot metal is only subjected to pressing. Under pressure, bronze is perfectly processed both cold and hot.
Beryllium bronze
This is an alloy belonging to the group of dispersion-hardening metals. It has high mechanical, physical and elastic properties.
Beryllium bronze has a high level of heat resistance, corrosion resistance and cyclic strength. It is resistant to low temperatures, does not magnetize and does not produce sparks upon impact.
Hardening of beryllium bronzes is carried out at temperatures of 750-790 degrees Celsius.