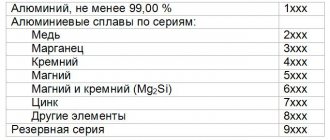
The relatively low resistivity of copper is an important, but not the only positive factor. The widespread use of this material is explained by its reasonable cost and resistance to adverse external influences. It is easy to create high-quality products of the required shape from it, which, without additional protection, retain functionality during long-term use in difficult conditions.
Various types of cable products are made from copper
Temperature coefficient of electrical resistivity of aluminum
Temperature coefficient of resistance
As you may have noticed, the electrical resistivity values in the table from the previous article are given at a temperature of 20 ° Celsius. If you assumed that they could change with temperature changes, you were right.
The dependence of wire resistance on temperature other than standard (usually 20 degrees Celsius) can be expressed through the following formula:
The constant "alpha" (α) is known as the temperature coefficient of resistance, which is equal to the relative change in the electrical resistance of a section of an electrical circuit or the resistivity of a substance when the temperature changes by one. Since all materials have a certain resistivity (at a temperature of 20 ° C), their resistance will change by a certain amount depending on the change in temperature. For pure metals, the temperature coefficient of resistance is a positive number, which means their resistance increases with increasing temperature. For elements such as carbon, silicon and germanium, this coefficient is a negative number, meaning their resistance decreases as temperature increases. Some metal alloys have a temperature coefficient of resistance very close to zero, meaning their resistance changes very little with temperature. The following table shows the temperature coefficients of resistance of several common types of metals:
| Conductor | α, per degree Celsius |
| Nickel | 0,005866 |
| Iron | 0,005671 |
| Molybdenum | 0,004579 |
| Tungsten | 0,004403 |
| Aluminum | 0,004308 |
| Copper | 0,004041 |
| Silver | 0,003819 |
| Platinum | 0,003729 |
| Gold | 0,003715 |
| Zinc | 0,003847 |
| Steel (alloy) | 0,003 |
| Nichrome (alloy) | 0,00017 |
| Nichrome V (alloy) | 0,00013 |
| Manganin (alloy) | 0,000015 |
| Constantan (alloy) | 0,000074 |
Let's use the example below to see how temperature can affect the resistance of the wires and its overall functioning:
The total resistance of the wires of this circuit (wire 1 + wire 2) at a standard temperature of 20 ° C is 30 ohms. Let's analyze the circuit using a table of voltages, currents and resistances:
At 20°C we get 12.5V across the load, and a total of 1.5V (0.75 + 0.75) voltage drop across the wire resistance. If the temperature is raised to 35 ° C, then using the above formula we can easily calculate the change in resistance on each of the wires. For copper wires (α = 0.004041) this change will be:
By recalculating the table values, we can see what consequences the temperature change led to:
Comparing these tables, we can come to the conclusion that the voltage across the load decreased with increasing temperature (from 12.5 to 12.42 volts), and the voltage drop across the wires increased (from 0.75 to 0.79 volts). The changes may seem minor at first glance, but they can be significant for long power lines connecting power plants and substations, substations and consumers.
Source
Applications of copper in electrical and electronic systems
In order to understand the reason for the popularity of copper as a material for the manufacture of elements of electrical and electronic systems, it is enough to look at the value of its resistivity in the table. For copper, this parameter is 0.0175 Ohm*mm2/meter. In this regard, copper is second only to silver.
It is the low resistivity, measured at a temperature of 20 degrees Celsius, that is the main reason that almost no electronic and electrical device can do without copper today. Copper is the main material for the production of wires and cables, printed circuit boards, electric motors and power transformer parts.
The low resistivity that copper is characterized by allows it to be used for the manufacture of electrical devices characterized by high energy-saving properties. In addition, the temperature of copper conductors increases very little when electric current passes through them.
Dependence of copper resistance on temperature
Thermophysical properties of aluminum alloys AMts, AMg, D16, AK, etc.
The table presents the composition and thermophysical properties of aluminum alloys for cold-worked, hardened and annealed states of the alloy:
- density of alloys, kg/m3;
- thermal conductivity coefficient, W/(m deg);
- coefficient of linear thermal expansion, 1/deg;
- specific electrical resistance, Ohm m.
Thermophysical properties are presented for the following aluminum alloys: A, AMts, AMg, Amg1, AMg5, AB, D18, D1, D16, AK8, AK4, 32S, V95. The properties of the alloys are given at room temperature, with the exception of the coefficient of thermal expansion (CTE), which is indicated for the temperature ranges of 20-100, 20-200 and 20-300°C.
Temperature dependence
Electrical resistivity depends on temperature. But all groups of substances manifest themselves differently when it changes. This must be taken into account when calculating wires that will operate under certain conditions. For example, on the street, where temperature values depend on the time of year, the necessary materials are less susceptible to changes in the range from -30 to +30 degrees Celsius. If you plan to use it in equipment that will operate under the same conditions, then you also need to optimize the wiring for specific parameters. The material is always selected taking into account the use. In the nominal table, electrical resistivity is taken at a temperature of 0 degrees Celsius. The increase in the indicators of this parameter when the material is heated is due to the fact that the intensity of the movement of atoms in the substance begins to increase. Electric charge carriers scatter randomly in all directions, which leads to the creation of obstacles to the movement of particles. The amount of electrical flow decreases.
As the temperature decreases, the conditions for current flow become better. Upon reaching a certain temperature, which will be different for each metal, superconductivity appears, at which the characteristic in question almost reaches zero.
The differences in parameters sometimes reach very large values. Those materials that have high performance can be used as insulators. They help protect wiring from short circuits and unintentional human contact. Some substances are not applicable at all for electrical engineering if they have a high value of this parameter. Other properties may interfere with this. For example, the electrical conductivity of water will not be of much importance for a given area. Here are the values of some substances with high indicators.
| High resistivity materials | ρ (Ohm m) |
| Bakelite | 1016 |
| Benzene | 1015…1016 |
| Paper | 1015 |
| Distilled water | 104 |
| Sea water | 0.3 |
| Dry wood | 1012 |
| The ground is wet | 102 |
| Quartz glass | 1016 |
| Kerosene | 1011 |
| Marble | 108 |
| Paraffin | 1015 |
| Paraffin oil | 1014 |
| Plexiglass | 1013 |
| Polystyrene | 1016 |
| Polyvinyl chloride | 1013 |
| Polyethylene | 1012 |
| Silicone oil | 1013 |
| Mica | 1014 |
| Glass | 1011 |
| Transformer oil | 1010 |
| Porcelain | 1014 |
| Slate | 1014 |
| Ebonite | 1016 |
| Amber | 1018 |
Substances with low performance are used more actively in electrical engineering. These are often metals that serve as conductors. There are also many differences between them. To find out the electrical resistivity of copper or other materials, it is worth looking at the reference table.
| Low resistivity materials | ρ (Ohm m) |
| Aluminum | 2.7·10-8 |
| Tungsten | 5.5·10-8 |
| Graphite | 8.0·10-6 |
| Iron | 1.0·10-7 |
| Gold | 2.2·10-8 |
| Iridium | 4.74·10-8 |
| Constantan | 5.0·10-7 |
| Cast steel | 1.3·10-7 |
| Magnesium | 4.4·10-8 |
| Manganin | 4.3·10-7 |
| Copper | 1.72·10-8 |
| Molybdenum | 5.4·10-8 |
| Nickel silver | 3.3·10-7 |
| Nickel | 8.7·10-8 |
| Nichrome | 1.12·10-6 |
| Tin | 1.2·10-7 |
| Platinum | 1.07·10-7 |
| Mercury | 9.6·10-7 |
| Lead | 2.08·10-7 |
| Silver | 1.6·10-8 |
| Gray cast iron | 1.0·10-6 |
| Carbon brushes | 4.0·10-5 |
| Zinc | 5.9·10-8 |
| Nikelin | 0,4·10-6 |
What does thermal conductivity depend on?
Studying the ability of heat transfer by metal products, it was revealed that thermal conductivity depends on:
- type of metal;
- chemical composition;
- porosity;
- sizes.
Metals have different crystal lattice structures, and this can change the thermal conductivity of the material. For example, in steel and aluminum, the structural features of microparticles affect differently the rate of transfer of thermal energy through them.
The thermal conductivity coefficient can have different values for the same metal when the exposure temperature changes. This is due to the fact that different metals have different melting degrees, which means that under other environmental parameters, the properties of the materials will also differ, and this will affect thermal conductivity.
Disadvantages of the high thermal conductivity of copper and its alloys
Copper has a much higher cost than brass or aluminum. At the same time, this metal has its disadvantages, which are directly related to its advantages. High thermal conductivity leads to the need to create special conditions during cutting, welding and soldering of copper elements. Since copper elements need to be heated much more concentrated compared to steel. Also, preliminary and concomitant heating of the part is often required.
Don’t forget that copper pipes require careful insulation if they make up the main line or distribution of the heating system. Which leads to an increase in the cost of network installation compared to options when other materials are used.
Example of thermal insulation of copper pipes
Difficulties also arise with gas welding of copper: this process will require more powerful torches. When welding metal 8–10 mm thick, two or three torches will be required. While one torch is used for welding, the other is heating the part. In general, welding work with copper requires increased costs for consumables.
It should also be said about the need to use special tools. So, to cut brass and bronze up to 15 cm thick, you will need a cutter capable of working with high-chromium steel 30 cm thick. Moreover, the same tool is enough to work with pure copper only 5 cm thick.
Thermal conductivity of aluminum alloys
A summary table of the thermal conductivity of aluminum alloys is presented. It shows the thermal conductivity values of common aluminum alloys (aluminum alloys with silicon, copper, magnesium and zinc, casting alloys, duralumin) at various temperatures in the range from 4 to 700K.
According to the table, it can be seen that the thermal conductivity of aluminum alloys mainly increases with increasing temperature. An alloy such as AD1 has the greatest thermal conductivity at room temperature - its thermal conductivity at this temperature is 210 W/(m deg). Lower thermal conductivity is characteristic mainly of cast aluminum alloys, for example AK4, AL1, AL8 and others.
The temperature in the table is in degrees Kelvin! Table of thermal conductivity of aluminum alloys
| Aluminium alloy | Temperature, K | Thermal conductivity of aluminum alloy, W/(m deg) |
| AB | 298…373…473…573 | 176…180…184…189 |
| AD1 cold-hardened | 4…10…20…40…80…150…300 | 50…130…260…400…250…220…210 |
| AD31 hardened, aged | 4…10…20…40…80…200…300…600 | 35…87…170…270…230…200…190…190 |
| AD33 | 300…373…473…573 | 140…151…163…172 |
| AD35 | 298…373…473…573 | 170…174…178…182 |
| AK4 | 300…500…600…700 | 145…160…170…170 |
| AK6 hardened, aged | 20…77…223…293…373…473…573…673 | 35…90…192…176…180…184…184…189 |
| AK8 hardened, aged | 20…40…80…150…300…573…673 | 50…72…100…125…160…180…180 |
| AL1 | 300…400…600 | 130…140…150 |
| AL2 | 20…77…293 | 10…18…160 |
| AL4 | 300…473…673 | 150…160…155 |
| AL5 | 300…473…573 | 160…170…180 |
| AL8 | 300…473…673 | 92…100…110 |
| AMg1 | 298…373…473…573…673 | 184…188…192…188…188 |
| AMg2 | 4…10…20…40…80…150…300…373…473…573…673 | 4,6…12…25…49…77…100…155…159…163…164…167 |
| AMg3 | 20…77…90…203…293 | 41…86…89…123…132 |
| AMg5 annealed | 10…20…40…80…150…300…473…673 | 10…20…40…66…92…130…130…150 |
| AMg6 | 20…77…173…293 | 13…43…75…92 |
| AMts cold-worked | 4…10…20…40…80…150…300…473…573…673 | 11…28…58…110…140…150…180…180…184…188 |
| B93 | 300…473…673 | 160…170…160 |
| B95 | 300…473…673 | 155…160…160 |
| VAD1 | 20…80…300 | 30…61…160 |
| VAL1 | 300…473…673 | 130…150…160 |
| VAL5 | 300…573…673 | 150…160…160 |
| VD17 | 300…673 | 130…170 |
| D1 | 298…373…473…573…673 | 117…130…150…172…176 |
| D16 hardened, aged | 10…20…40…80…150…300…373…473…573 | 9…19…37…61…90…120…130…146…163 |
| D20 hardened, aged | 20…40…80…150…300…373…473…573…673 | 27…38…61…85…140…142…147…155…160 |
| D21 | 298…373…473…573 | 130…138…151…168 |
Characteristics of thermal conductivity of materials
The concept of thermal conductivity of materials is characterized by the ability to transfer thermal energy within a certain object from heated parts to cold ones. The process is carried out by atoms, molecules, electrons and occurs in any body with an uneven temperature distribution.
From the standpoint of kinetic physics, this process occurs as a result of the interaction of particles of molecules in hotter areas within the sample with other elements characterized by a lower temperature. The mechanism and rate of heat transfer depends on the state of aggregation of the substance.
The thermal conductivity category involves determining the heating rate of a material sample and the movement of a temperature wave in a certain direction. The indicator depends on physical parameters:
- density;
- temperature of phase transition to liquid state
- speed of sound propagation (for dielectrics).
Thermal conductivity of brass and bronze
The table shows the thermal conductivity values of brass, bronze, as well as copper-nickel alloys (constantan, copel, manganin, etc.) depending on temperature - in the range from 4 to 1273 K.
The thermal conductivity of brass, bronze and other copper-based alloys increases when heated. According to the table, L96 brass has the highest thermal conductivity of the alloys considered at room temperature . Its thermal conductivity at a temperature of 300 K (27°C) is 244 W/(m deg).
Also copper alloys with high thermal conductivity include: brass LS59-1, tombac L96 and L90, tin tombac LTO90-1, rolled tombac RT-90. In addition, the thermal conductivity of brass is generally higher than that of bronze. It should be noted that bronzes with high thermal conductivity include: phosphorus, chromium and beryllium bronzes, as well as BrA5 bronze.
The copper alloy with the lowest thermal conductivity is manganese bronze - its thermal conductivity coefficient at a temperature of 27°C is 9.6 W/(m deg).
The thermal conductivity of copper alloys is always lower than the thermal conductivity of pure copper, all other things being equal. In addition, the thermal conductivity of copper-nickel alloys is particularly low. The most thermally conductive of them at room temperature is cupronickel MNZhMts 30-0.8-1 with a thermal conductivity of 30 W/(m deg).
Table of thermal conductivity of brass, bronze and copper-nickel alloys Alloy Temperature, KThermal conductivity, W/(m deg) Copper-nickel alloys Brass Bronze
| Beryllium copper | 300 | 111 |
| Constantan of foreign production | 4…10…20…40…80…300 | 0,8…3,5…8,8…13…18…23 |
| Constantan MNMts40-1.5 | 273…473…573…673 | 21…26…31…37 |
| Kopel MNMts43-0.5 | 473…1273 | 25…58 |
| Manganin of foreign production | 4…10…40…80…150…300 | 0,5…2…7…13…16…22 |
| Manganin MNMts 3-12 | 273…573 | 22…36 |
| Cupronickel MNZHMts 30-0.8-1 | 300 | 30 |
| Nickel silver | 300…400…500…600…700 | 23…31…39…45…49 |
| Automatic brass UNS C36000 | 300 | 115 |
| L62 | 300…600…900 | 110…160…200 |
| L68 deformed brass | 80…150…300…900 | 71…84…110…120 |
| L80 semi-tompak | 300…600…900 | 110…120…140 |
| L90 | 273…373…473…573…673…773…873 | 114…126…142…157…175…188…203 |
| L96 tombak drawn | 300…400…500…600…700…800 | 244…245…246…250…255…260 |
| LAN59-3-2 aluminum-nickel brass | 300…600…900 | 84…120…150 |
| LMC58-2 manganese brass | 300…600…900 | 70…100…120 |
| LO62-1 tin | 300 | 99 |
| LO70-1 tin | 300…600 | 92…140 |
| LS59-1 annealed brass | 4…10…20…40…80…300 | 3,4…10…19…34…54…120 |
| LS59-1V leaded brass | 300…600…900 | 110…140…180 |
| LTO90-1 tombak tin | 300…400…500…600…700…800…900 | 124…141…157…174…194…209…222 |
| BrA5 | 300…400…500…600…700…800…900 | 105…114…124…133…141…148…153 |
| BrA7 | 300…400…500…600…700…800…900 | 97…105…114…122…129…135…141 |
| BrAZhMC10-3-1.5 | 300…600…800 | 59…77…84 |
| BrAZHN10-4-4 | 300…400…500 | 75…87…97 |
| BrAZHN11-6-6 | 300…400…500…600…700…800 | 64…71…77…82…87…94 |
| BrB2, annealed at 573K | 4…10…20…40…80 | 2,3…5…11…21…37 |
| BrKd | 293 | 340 |
| BrKMTs3-1 | 300…400…500…600…700 | 42…50…55…54…54 |
| BrMC-5 | 300…400…500…600…700 | 94…103…112…122…127 |
| BrMTsS8-20 | 300…400…500…600…700…800…900 | 32…37…43…46…49…51…53 |
| BrO10 | 300…400…500 | 48…52…56 |
| BrOS10-10 | 300…400…600…800 | 45…51…61…67 |
| BrOS5-25 | 300…400…500…600…700…800…900 | 58…64…71…77…80…83…85 |
| BrOF10-1 | 300…400…500…600…700…800…900 | 34…38…43…46…49…51…52 |
| BrOTs10-2 | 300…400…500…600…700…800…900 | 55…56…63…68…72…75…77 |
| BrOTs4-3 | 300…400…500…600…700…800…900 | 84…93…101…108…114…120…124 |
| BrOTs6-6-3 | 300…400…500…600…700…800…900 | 64…71…77…82…87…91…93 |
| BrOTs8-4 | 300…400…500…600…700…800…900 | 68…77…83…88…93…96…100 |
| Aluminum bronze | 300 | 56 |
| Aged beryllium bronze | 20…80…150…300 | 18…65…110…170 |
| Manganese bronze | 300 | 9,6 |
| Production leaded bronze | 300 | 26 |
| Phosphor bronze 10% | 300 | 50 |
| Phosphor bronze annealed | 20…80…150…300 | 6…20…77…190 |
| Chromium bronze UNS C18200 | 300 | 171 |
Metal characteristics
The melting temperature of brass, depending on its composition, ranges from 880-950°C. Thus, with an increase in zinc impurity in the material under consideration, the melting point will decrease. It is worth noting that brass, due to its properties, can be welded well.
Brass is processed by resistance welding and can be rolled. The uncoated surfaces of the metal in question turn black when in contact with air. Brass has a yellow color and is highly polished. The non-ferrous metal in question can be melted within certain temperature limits, depending on the impurities in the composition of the material.
Metal specifications:
- Melting point – 880-950°C;
- Material density – 8,300-8,700 kg/cubic meter;
- Specific heat capacity - 0.377 kJ kg−1 K−1 at 20°C;
- Electrical resistivity - (0.07-0.08)·10−6 Ohm·m.
It is useful to know that bismuth, as well as lead, have a detrimental effect on brass, since they reduce the ability to deform when hot.
What are the advantages of non-ferrous metal, grade and application?
Brass belongs to the category of non-ferrous metals. It is useful to know about the chemical and physical benefits that brass has.
Advantages:
- Corrosion resistance;
- High degree of fluidity;
- Excellent anti-friction properties;
- Slight tendency to segregation;
- Excellent technological properties;
- Excellent mechanical properties.
The list presented above does not limit the advantages and beneficial properties of this metal. You should not ignore the most popular brands of material, as well as application.
Melting point of brass
The melting point of brass of the considered brands varies in the range from 865 to 1055 °C. The most fusible is manganese brass LMts58-2 with a melting point of 865°C. Low-melting brasses also include: L59, L62, LAN59-3-2, LKS65-1.5-3 and others.
L96 brass has the highest melting point (1055°C). Among the refractory brasses, according to the table, we can also distinguish: brass L90, LA85-0.5, tin tombak LTO90-1.
Melting temperature of brass Brass, °СBrass, °С
| L59 | 885 | LMts55-3-1 | 930 |
| L62 | 898 | LMts58-2 manganese brass | 865 |
| L63 | 900 | LMtsA57-3-1 | 920 |
| L66 | 905 | LMtsZh52-4-1 | 940 |
| L68 deformed brass | 909 | LMtsOS58-2-2-2 | 900 |
| L70 | 915 | LMtsS58-2-2 | 900 |
| L75 | 980 | LN56-3 | 890 |
| L80 semi-tompak | 965 | LN65-5 | 960 |
| L85 | 990 | LO59-1 | 885 |
| L90 | 1025 | LO60-1 | 885 |
| L96 tombak drawn | 1055 | LO62-1 tin | 885 |
| LA67-2.5 | 995 | LO65-1-2 | 920 |
| LA77-2 | 930 | LO70-1 tin | 890 |
| LA85-0.5 | 1020 | LO74-3 | 885 |
| LAZ60-1-1 | 904 | LO90-1 | 995 |
| LAZHMts66-6-3-2 | 899 | LS59-1 | 900 |
| LAN59-3-2 aluminum-nickel brass | 892 | LS59-1V leaded brass | 900 |
| LANKMts75-2-2.5-0.5-0.5 | 940 | LS60-1 | 900 |
| LZhMts59-1-1 | 885 | LS63-3 | 885 |
| LK80-3 | 900 | LS64-2 | 910 |
| LKS65-1.5-3 | 870 | LS74-3 | 965 |
| LKS80-3-3 | 900 | LTO90-1 tombak tin | 1015 |
Melting point of bronze
The melting point of bronze ranges from 854 to 1135°C. Bronze AZHN11-6-6 has the highest melting point - it melts at a temperature of 1408 K (1135°C). The melting point of this bronze is even higher than the melting point of copper, which is 1084.6°C.
Bronzes with a low melting point include: BrOTs8-4, BrB2, BrMTsS8-20, BrSN60-2.5 and the like.
Melting point of bronze Bronzet, °СBronzet, °С
| BrA5 | 1056 | BrOS8-12 | 940 |
| BrA7 | 1040 | BrOSN10-2-3 | 1000 |
| BrA10 | 1040 | BrOF10-1 | 934 |
| BrAZH9-4 | 1040 | BrOF4-0.25 | 1060 |
| BrAZhMC10-3-1.5 | 1045 | BrOTs10-2 | 1015 |
| BrAZHN10-4-4 | 1084 | BrOTs4-3 | 1045 |
| BrAZHN11-6-6 | 1135 | BrOTs6-6-3 | 967 |
| BrAZhS7-1.5-1.5 | 1020 | BrOTs8-4 | 854 |
| BrAMTS9-2 | 1060 | BrOTsS3.5-6-5 | 980 |
| BrB2 | 864 | BrOTsS4-4-17 | 920 |
| BrB2.5 | 930 | BrOTsS4-4-2.5 | 887 |
| BrKMTs3-1 | 970 | BrOTsS5-5-5 | 955 |
| BrKN1-3 | 1050 | BrOTsS8-4-3 | 1015 |
| BrKS3-4 | 1020 | BrOTsS3-12-5 | 1000 |
| BrKTs4-4 | 1000 | BrOTsSN3-7-5-1 | 990 |
| BrMG0.3 | 1076 | BrS30 | 975 |
| BrMC5 | 1007 | BrSN60-2.5 | 885 |
| BrMTsS8-20 | 885 | BrSUN7-2 | 950 |
| BrO10 | 1020 | BrХ0.5 | 1073 |
| BrOS10-10 | 925 | BrTsr0.4 | 965 |
| BrOS10-5 | 980 | Cadmium | 1040 |
| BrOS12-7 | 930 | Silver | 1082 |
| BrOS5-25 | 899 | HOT alloy | 1075 |
Note: The melting and boiling points of other common metals are given in this table.
Thermal conductivity of non-ferrous metals and technical alloys
The table shows the thermal conductivity values of metals (non-ferrous), as well as the chemical composition of metals and technical alloys in the temperature range from 0 to 600°C.
Non-ferrous metals and alloys: nickel Ni, monel, nichrome; nickel alloys (according to GOST 492-58): cupronickel NM81, NM70, constantan NMMts 58.5-1.54, copel NM 56.5, monel NMZhMts and K-monel, alumel, chromel, manganin NMMts 85-12, invar; magnesium alloys (according to GOST 2856-68), electron, platinum-rhodium; soft solders (according to GOST 1499-70): pure tin, lead, POS-90, POS-40, POS-30, Rose alloy, Wood alloy.
The table shows that magnesium alloys and nickel have high thermal conductivity (at room temperature). Low thermal conductivity is characteristic of nichrome, invar and Wood's alloy.
Thermal conductivity coefficients of alloys
The table shows the thermal conductivity values of alloys in the temperature range from 20 to 200ºС. Alloys: aluminum bronze, bronze, phosphor bronze, invar, constantan, manganin, magnesium alloys, copper alloys, Rose alloy, Wood's alloy, nickel alloys, nickel silver, platinum-iridium, electron alloy, platinum-rhodium.
Thermal conductivity coefficient of other materials
MaterialHumidity mass fraction % W/(m•K)
| Bakelite varnish | — | 0,29 |
| Concrete with crushed stone | 8 | 1,28 |
| Plain paper | Air dry | 0,14 |
| Viniplast | — | 0,13 |
| Gravel | Air dry | 0,36 |
| Granite | — | 3,14 |
| Clay | 15-20 | 0,7-0,93 |
| Oak (along the grain) | 6-8 | 0,35-0,43 |
| Oak (across the grain) | 6-8 | 0,2-0,21 |
| Reinforced concrete | 8 | 1,55 |
| Cardboard | Air dry | 0,14-0,35 |
| Brickwork | Air dry | 0,67-0,87 |
| Leather | >> | 0,14-0,16 |
| Ice | — | 2,21 |
| Cork boards | 0 | 0,042-0,054 |
| Freshly fallen snow | — | 0,105 |
| Snow compacted | — | 0,35 |
| The snow has begun to melt | — | 0,64 |
| Pine (along the grain) | 8 | 0,35-0,41 |
| Pine (across the grain) | 8 | 0,14-0,16 |
| Glass (ordinary) | — | 0,74 |
| Ftoroplast-3 | — | 0,058 |
| Ftoroplast-4 | — | 0,233 |
| Cinder concrete | 13 | 0,698 |
| Plaster | 6-8 | 0,791 |
Thermal conductivity coefficient of asbestos and foam concrete at different temperatures
(ρa=576kg/m3, ρп=400kg/m3,λ, W/(m•K))
Material-18oС0oС50oС100oС150oС
| Asbestos | — | 0,15 | 0,18 | 0,195 | 0,20 |
| Foam concrete | 0,1 | 0,11 | 0,11 | 0,13 | 0,17 |
Thermal conductivity coefficient of liquid W/(m•K) at different temperatures
Material0oС50oС100oС
| Aniline | 0,19 | 0,177 | 0,167 |
| Acetone | 0,17 | 0,16 | 0,15 |
| Benzene | — | 0,138 | 0,126 |
| Water | 0,551 | 0,648 | 0,683 |
| Vaseline oil | 0,126 | 0,122 | 0,119 |
| Castor oil | 0,184 | 0,177 | 0,172 |
| Methyl alcohol | 0,214 | 0,207 | — |
| Ethanol | 0,188 | 0,177 | — |
| Toluene | 0,142 | 0,129 | 0,119 |
Specific resistance and temperature coefficient of expansion (CTE) of metal wire (at 18ºС)
The table shows the values of electrical resistivity and CTE of metal wire made of various metals and alloys. Wire material: aluminum, tungsten, iron, gold, brass, manganin, copper, nickel, constantan, nichrome, tin, platinum, lead, silver, zinc. As can be seen from the table, nichrome wire has a high electrical resistivity and is successfully used as incandescent heating coils for many household and industrial devices.
Specific heat capacity of multicomponent special alloys
The specific (mass) heat capacity of multicomponent special alloys is given in the table at temperatures from 0 to 1300ºС. Heat capacity dimension cal/(g deg). Heat capacity of special alloys: alumel, bell metal, Wood's alloy, Invar, Lipowitz alloy, Manganin, Monel, Rose alloy, phosphorus bronze, chromel, Na-K alloy, Pb - Bi alloy, Pb - Bi - Sn, Zn - Sn - Ni - Fe - Mn.
Resistivity of metals, electrolytes and substances (Table)
Resistivity of metals and insulators
The reference table gives the values of resistivity p of some metals and insulators at a temperature of 18-20 ° C, expressed in ohm cm. The value of p for metals strongly depends on impurities; the table shows the values of p for chemically pure metals, and for insulators they are given approximately. Metals and insulators are arranged in the table in order of increasing p .
Importance in everyday life and production
Why is it important to consider thermal conductivity? A similar value is indicated in various tables for each metal and is taken into account in the following cases:
- In the manufacture of various heat exchangers. Heat is one of the important carriers of energy. It is used to provide comfortable living conditions in residential and other premises. When creating heating radiators and boilers, it is important to ensure rapid and complete heat transfer from the coolant to the end consumer.
- In the manufacture of outlet elements. You can often encounter a situation where you need to remove heat rather than supply it. An example is the case of heat removal from the cutting edge of a tool or gear teeth. To ensure that the metal does not lose its basic performance qualities, rapid removal of thermal energy is ensured.
- When creating insulating layers. In some cases, the material should not conduct thermal energy transfer. For such operating conditions, a metal is selected that has a low heat conductivity coefficient.
The indicator under consideration is determined when testing under various conditions. As previously noted, the thermal conductivity coefficient may depend on the operating temperature. Therefore, the tables indicate several of its values.
Properties of aluminum alloys with silicon, copper, magnesium and zinc
The table presents the composition and the following thermophysical properties of aluminum alloys:
- density of alloys, kg/m3;
- thermal conductivity coefficient, W/(m °C);
- coefficient of linear thermal expansion, 1/deg;
- corrosion resistance in water and air;
- temperature of strength change.
The density, thermal conductivity and coefficient of linear thermal expansion of the alloys are presented as a function of temperature in the range from 500 to 660°C. The density of aluminum alloys with silicon and zinc is the highest. Light alloys include alloys containing magnesium.
It should be noted that aluminum alloys with a high copper content have the greatest corrosion resistance in water and air - they are resistant to corrosion up to temperatures of 200...250°C. Such alloys also have high strength characteristics.
Is it possible to increase the thermal conductivity of copper?
Copper is widely used in the creation of microcircuits for electronic devices and is designed to remove heat from parts heated by electric current. When trying to increase the speed of modern computers, developers were faced with the problem of cooling processors and other parts. One of the solutions was to split the processor into several cores. However, this method of combating overheating has exhausted itself, and now it is necessary to look for new conductors with higher thermal and electrical conductivity.
One solution to this problem is the recently discovered element graphene. Thanks to graphene deposition, the thermal conductivity of the copper element increases by 25%. However, the invention is still at the development level.
High conductivity materials
The most widespread materials of high conductivity include copper and aluminum (Superconducting materials, which have a typical resistance 10-20 times lower than ordinary conductive materials (metals), are discussed in the section Superconductivity).
The advantages of copper, which ensure its widespread use as a conductor material, are as follows:
- low resistivity;
- sufficiently high mechanical strength;
- corrosion resistance is satisfactory in most applications;
- good workability: copper is rolled into sheets, strips and drawn into wire, the thickness of which can be increased to thousandths of a millimeter;
- relative ease of soldering and welding.
Copper is most often obtained by processing sulfide ores. After a series of ore smelting and roasting with intense blasting, copper intended for electrical purposes must undergo a process of electrolytic purification.
Copper grades M1 and M0 are most often used as conductor material. M1 grade copper contains 99.9% Cu, and in the total amount of impurities (0.1%) oxygen should be no more than 0.08%. The presence of oxygen in copper worsens its mechanical properties. The best mechanical properties are found in M0 grade copper, which contains no more than 0.05% impurities, including no more than 0.02% oxygen.
Copper is a relatively expensive and scarce material, so it is increasingly being replaced by other metals, especially aluminum.
In some cases, alloys of copper with tin, silicon, phosphorus, beryllium, chromium, magnesium, and cadmium are used. Such alloys, called bronzes, with the correct composition, have significantly higher mechanical properties than pure copper.
Aluminum
Aluminum is the second most important conductor material after copper. This is the most important representative of the so-called light metals: the density of cast aluminum is about 2.6, and rolled aluminum is 2.7 Mg/m3. Thus, aluminum is approximately 3.5 times lighter than copper. The temperature coefficient of expansion, specific heat capacity and heat of fusion of aluminum are greater than those of copper. Due to the high values of specific heat capacity and heat of fusion, heating aluminum to the melting point and transferring it to a molten state requires more heat than heating and melting the same amount of copper, although the melting point of aluminum is lower than that of copper.
Aluminum has lower properties compared to copper - both mechanical and electrical. With the same cross-section and length, the electrical resistance of an aluminum wire is 1.63 times greater than that of a copper wire. It is very important that aluminum is less scarce than copper.
For electrical purposes, aluminum containing no more than 0.5% impurities, grade A1, is used. Even purer AB00 grade aluminum (no more than 0.03% impurities) is used for the manufacture of aluminum foil, electrodes and housings of electrolytic capacitors. Aluminum of the highest purity AB0000 has an impurity content of no more than 0.004%. Additives of Ni, Si, Zn or Fe at a content of 0.5% reduce the γ of annealed aluminum by no more than 2-3%. A more noticeable effect is exerted by Cu, Ag and Mg impurities, which, at the same mass content, reduce γ aluminum by 5-10%. Ti and Mn greatly reduce the electrical conductivity of aluminum.
Aluminum oxidizes very actively and becomes covered with a thin oxide film with high electrical resistance. This film protects the metal from further corrosion.
Aluminum alloys have increased mechanical strength. An example of such an alloy is Aldrey , containing 0.3-0.5% Mg, 0.4-0.7% Si and 0.2-0.3% Fe. In aldrey, the Mg2Si compound is formed, which imparts high mechanical properties to the alloy.
Resistivity of copper of various grades
Round copper wire for wires, cables, etc. can be soft (MM grade), hard (MT grade) and MS grade. It is produced in the diameter range of 0.02-9.42 mm. The electrical resistivity of the wire to direct current at 20℃ corresponds to the values given in the table:
| Wire diameter, mm | ρ at 20℃, µOhm-m | |
| MM | MT, MS | |
| Less than 1.00 | — | 0,018 |
| 1,0-2,44 | 0,01724 | 0,0178 |
| 2.50 and more | — | 0,0177 |
The advantages of copper in terms of conductivity give rise to its widespread use in the production of conductors. However, copper is a relatively expensive and scarce material, so it is increasingly being replaced by other metals, including aluminum.
You might be interested in the principle of operation of current relays and types of devices
The wire
Alloys of copper with tin, chromium, cadmium and others are called bronzes. With the correct selection of composition, bronze differs very favorably from pure copper in terms of mechanical properties.
General characteristics of copper
Speaking about copper, it must be said that at the dawn of the electrical era it began to be used in the production of electrical equipment. It began to be used largely due to the unique properties that this alloy has. By itself, it is a material characterized by high properties in terms of ductility and good malleability.
Along with the thermal conductivity of copper, one of its most important advantages is its high electrical conductivity. It is thanks to this property that copper has become widespread in power plants , in which it acts as a universal conductor. The most valuable material is electrolytic copper, which has a high degree of purity of 99.95%. Thanks to this material, it becomes possible to produce cables.
This is interesting: Performance characteristics and advantages of copper pipes and fittings for plumbing
Density, thermal conductivity, heat capacity of aluminum alloys Amts, Amg1, Amg2, D1, D16
The values of density (at a temperature of 293 K), thermal conductivity coefficient, W/(m °C), and specific (mass) heat capacity, kJ/(kg °C) of some aluminum alloys are presented depending on temperature (properties are given at temperatures of 25. 100, 200, 300, 400 °C).
The table shows the density, thermal conductivity, and heat capacity of the following aluminum alloys: Amts, Amg1, Amg2, D1, D16. It should be noted that the density of aluminum alloys is approximately the same, but aluminum alloy such as D-1 stands out a little - its density is 2800 kg/m3.
Application
The state of aggregation of materials has a distinctive structure of molecules and atoms. This is what has a great influence on metal products and their properties, depending on their purpose.
The different chemical composition of components and parts made of iron allows them to have different thermal conductivities. This is due to the structure of metals such as cast iron, steel, copper and aluminum. The porosity of cast iron products promotes slow heating, and the density of the copper structure, on the contrary, accelerates the heat transfer process. These properties are used for rapid heat removal or gradual heating of inert products. An example of using the properties of metal products is:
- kitchen utensils with various properties;
- pipe soldering equipment;
- irons;
- rolling and sliding bearings;
- plumbing equipment for heating water;
- heating devices.
Copper tubes are widely used in radiators of automobile cooling systems and air conditioners used in everyday life. Cast iron radiators retain heat in the apartment, even with an inconsistent supply of coolant at the required temperature. And radiators made of aluminum contribute to the rapid transfer of heat to the heated room.
When high temperatures occur as a result of friction of metal surfaces, it is also important to take into account the thermal conductivity of the product. In any gearbox or other mechanical equipment, the ability to remove heat will allow the mechanism parts to maintain strength and not be subject to destruction during operation. Knowledge of the heat transfer properties of various materials will allow you to competently use certain alloys of non-ferrous or ferrous metals.
Sources
- https://tokar.guru/metally/stal/teploprovodnost-stali-alyuminiya-latuni-medi.html
- https://thermalinfo.ru/svojstva-materialov/metally-i-splavy/svojstva-medi-plotnost-teploemkost-teploprovodnost
- https://smolgelios.ru/provodka/teplo-elektroprovodnost-medi.html
- https://ometalledo.ru/teploprovodnost-medi-i-alyuminiya-tablica.html
- https://vse-otoplenie.ru/teplootdaca-aluminia
- https://thermalinfo.ru/svojstva-materialov/metally-i-splavy/teploprovodnost-splavov-medi-temperatura-plavleniya-bronzy-i-latuni
- https://kangen.ru/raznoe/temperatura-plavleniya-latuni-v-domashnih-usloviyah.html
- https://thermalinfo.ru/svojstva-materialov/metally-i-splavy/teploprovodnost-metallov-teploemkost-i-plotnost-splavov
- https://zaozmi.ru/polezno/tablica_teploprovodimosti_metallov.html
- https://tpspribor.ru/vidy-metalla/teploprovodnost-medi-dve-storony-odnoy-medali.html
- https://ectrl.ru/provodka/elektro-i-teploprovodnost-medi.html
- https://met-all.org/cvetmet-splavy/med/teploprovodnost-medi-i-ee-splavov.html
- https://prompriem.ru/metally/teploprovodnost.html
[collapse]
Thermal conductivity, heat capacity and resistivity of alloy 1151T
The table shows the values of the thermal conductivity coefficient, W/(m deg), specific (mass) heat capacity, kJ/(g deg) and resistivity of aluminum alloy 1151T.
The properties of aluminum alloy 1151T are given depending on temperature (in the range from 0 to 400 ° C). According to the table, it can be seen that the thermal conductivity of this alloy increases when heated, but around a temperature of 200°C there is a slight decrease followed by an increase. The same nature of change is characteristic of the specific heat capacity of the 1151T alloy. The electrical resistivity of the alloy in question increases as its temperature increases.
Copper is the main material for conductors
The resistivity of a substance is calculated using the formula, where one of the important indicators is the temperature coefficient of electrical resistance. The table contains the resistivity values of three known metals in the temperature range from 0 to 100°C.
If we take the resistivity of iron, as one of the available materials, equal to 0.1 Ohm, then for 1 Ohm you will need 10 meters. Silver has the lowest electrical resistance; for its value of 1 ohm it will be 66.7 meters. A significant difference, but silver is an expensive metal that is not practical to use everywhere. The next best indicator is copper, where 57.14 meters are required per 1 ohm. Due to its availability and cost compared to silver, copper is one of the popular materials for use in electrical networks. The low resistivity of copper wire or the resistance of copper wire makes it possible to use copper conductor in many branches of science, technology, as well as for industrial and domestic purposes.
Resistivity of copper: what is it equal to, table of material resistance
The directed movement of particles in any substance creates an electric current due to the formation of a potential difference. The individual physical characteristics of each substance determine its effect on the passage of current and are measured as electrical resistance.
The essence of the phenomenon
This is a value characteristic of a conductor having a length of 1 meter and a cross-sectional area of 1 square meter/millimeter. It is denoted by the Greek letter ρ. Different materials have different resistivities.
At the same time, the resistance of the conductor will change in direct proportion to the length and in inverse proportion to the cross-sectional area. That is, the longer the conductor, the higher it is, but the greater the thickness, the lower it is.
Units
Of practical importance in technology is a unit equal to a millionth of an ohm multiplied by a meter (Ohm-m), since even meeting a wire with a cross-section equal to one square meter or more is quite problematic. Therefore, a microohm meter (μOhm-m) is usually used in measurements:
1 µOhm-m = 1×10-6 Ohm-m = 1 Ohm-mm2/m
Formula for calculating resistivity
, where R is the conductor resistance (Ohm); L—conductor length (m); S - conductor cross-section (mm2).
Thus, ρ of a single-component piece of wire, the length of which is 1 meter, and the cross-sectional area is 1 square millimeter, with R equal to 1 ohm, will be 1 Ohm-mm2/m.
Table of electrical resistivity of some metals
| Wire type | ρ at 20℃, Ohm-m |
| Silver | 1,59×10⁻⁸ |
| Copper | 1,67×10⁻⁸ |
| Gold | 2,35×10⁻⁸ |
| Aluminum | 2,65×10⁻⁸ |
| Tungsten | 5,65×10⁻⁸ |
| Nickel | 6,84×10⁻⁸ |
| Iron | 9,7×10⁻⁸ |
| Platinum | 1,06×10⁻⁷ |
| Steel | 1,6×10⁻⁷ |
| Lead | 2,06×10⁻⁷ |
| Duralumin | 4,0×10⁻⁷ |
| Nichrome | 1,05×10⁻⁶ |
The resistivity is absolutely independent of the shape and size of the conductor, but varies over a wide range when the temperature deviates from the standard value of 20 degrees Celsius. Practical electrical engineering has proven that an increase in temperature increases the resistance of metals to current flow; on the other hand, along with a decrease in temperature, it decreases. It is possible to approximately calculate how significant the change will be, taking into account the fact that all metals have almost the same level of increase in loss of a given value, on average 0.4% per 1°C.
If this indicator needs to be determined accurately, then you can use this formula:
Table of electrical resistivity of some metals
| Wire type | ρ at 20℃, Ohm-m |
| Silver | 1,59×10⁻⁸ |
| Copper | 1,67×10⁻⁸ |
| Gold | 2,35×10⁻⁸ |
| Aluminum | 2,65×10⁻⁸ |
| Tungsten | 5,65×10⁻⁸ |
| Nickel | 6,84×10⁻⁸ |
| Iron | 9,7×10⁻⁸ |
| Platinum | 1,06×10⁻⁷ |
| Steel | 1,6×10⁻⁷ |
| Lead | 2,06×10⁻⁷ |
| Duralumin | 4,0×10⁻⁷ |
| Nichrome | 1,05×10⁻⁶ |
The resistivity is absolutely independent of the shape and size of the conductor, but varies over a wide range when the temperature deviates from the standard value of 20 degrees Celsius. Practical electrical engineering has proven that an increase in temperature increases the resistance of metals to current flow; on the other hand, along with a decrease in temperature, it decreases. It is possible to approximately calculate how significant the change will be, taking into account the fact that all metals have almost the same level of increase in loss of a given value, on average 0.4% per 1°C.
You may be interested in this All about free energy
Resistance chart
If this indicator needs to be determined accurately, then you can use this formula:
, where ρ and ρ0 are, respectively, resistivities at temperatures t and t (20°C, table value), α is the temperature coefficient of resistance.
| Wire type | α |
| Nickel | 0,005866 |
| Iron | 0,005671 |
| Molybdenum | 0,004579 |
| Tungsten | 0,004403 |
| Aluminum | 0,004308 |
| Copper | 0,004041 |
| Silver | 0,003819 |
| Platinum | 0,003729 |
| Gold | 0,003715 |
| Zinc | 0,003847 |
| Steel | 0,003 |
| Nichrome | 0,00017 |
So, for example, having found in the tables the resistivity of copper at 20 degrees Celsius and its temperature coefficient, you can calculate that when heated to 100℃ its resistance will increase by 32%. Almost the same thing will happen with the resistivity of an aluminum cable with the same coefficient (0.004). But the resistivity of steel will increase less significantly - by 24%.
Heat
As the temperature increases, the conductor is saturated with thermal energy, which is transferred to all atoms of the substance. This causes an increase in the intensity of their thermal movement. The latter factor leads to an increase in resistance to the movement of free electrons in a certain direction, since the probability of free electrons meeting atoms increases. When the temperature decreases, fewer atoms can impede the directional movement of electrons, hence the opposite occurs. As a result of a colossal drop in temperature, an interesting phenomenon occurs, called “superconductivity of metals”: resistance decreases to zero in conditions close to absolute zero (-273.15℃). In such conditions, the metal atoms freeze in their positions, and the electrons move without any obstacles.
Superconductivity
Thermophysical properties of aluminum alloys of the Al-Cu-Mn system
The table shows the thermophysical properties of aluminum alloys containing copper and manganese. such alloys as alloy 01205, 1201, D21, D20 were considered. The properties of the alloys are presented depending on the temperature in the range from 25 to 400°C. Of the alloys considered, the most thermally conductive is the D20 alloy, with a thermal conductivity of 138 W/(m deg) at a temperature of 25°C.
The following thermophysical properties of the alloys are given:
- thermal conductivity coefficient, W/(m deg);
- specific (mass) heat capacity, kJ/(kg deg);
- coefficient of linear thermal expansion, 1/deg.
What is thermal conductivity
This term means the ability of various materials to exchange energy , which in this case is represented by heat. In this case, energy transfer passes from the hotter part to the colder part and occurs due to:
- Molecules
- Atoms.
- Electrons and other particles of the metal structure.
The thermal conductivity of stainless steel will differ significantly from that of another metal - for example, the thermal conductivity of copper will be different than that of steel.
To indicate this indicator, a special value is used, called the thermal conductivity coefficient. It is characterized by the amount of heat that can pass through a material in a certain unit of time.
Thermal conductivity of high-strength aluminum alloys V93, alloy 1933, V95, alloy 1973, V96, etc.
The table shows the thermal conductivity values in the dimension W/(m deg) depending on the temperature (range from 25 to 400°C) of the following aluminum alloys: V93, V93pch, alloy 1933, V95, V95pch, V95och, alloy 1973, V96Ts, V96Ts -3. The most thermally conductive, according to the table, are alloys V93, V93pch, alloy 1933, having a thermal conductivity value of 163 W/(m deg) at a temperature of 25°C.
Sources: 1. Physical quantities. Directory. A.P. Babichev, N.A. Babushkina, A.M. Bratkovsky and others; Ed. I.S. Grigorieva, E.Z. Meilikhova. - M.: Energoatomizdat, 1991. - 1232 p. 2. Chirkin V.S. Thermophysical properties of nuclear technology materials. 3. V.M. Beletsky, G.A. Krivov. Aluminum alloys (composition, properties, technology, application). Directory. Under the general editorship. Academician of the Russian Academy of Sciences I.N. Fridlyandera - K.: "Comintekh", 2005. - 365 p. 4. Bogdanov S.N., Burtsev S.I., Ivanov O.P., Kupriyanova A.V. Refrigeration equipment. Air conditioning. Properties of substances: Reference / Ed. S.N. Bogdanov. 4th ed., revised. and additional - St. Petersburg: SPbGAKhPT, 1999. - 320 p.
What is electrical resistance?
It can be defined based on two positions. The first is related to the formula for Ohm's law. And it sounds like this: electrical resistance is a physical quantity that is defined as the ratio of the voltage in a conductor to the current flowing in it. The mathematical notation is given below.
The second is based on the properties of the body. The electrical resistance of a conductor is a physical quantity that indicates the ability of a body to convert electrical energy into heat. Both of these statements are true. Only in the school course they most often stop at memorizing the first. The quantity being studied is designated by the letter R. The units in which electrical resistance is measured are Ohms.
Source