Hardening is a widely used heat treatment technology for steel products. Its essence is to heat the metal so that its temperature reaches a critical point, at which changes in the crystal structure occur or the process of phase dissolution begins to occur in the matrix formed at low temperatures of the part. After this, the metal cools sharply. As a result, the steel acquires a needle-type microstructure called martensite. Thanks to this phenomenon, the hardness of the alloy increases and its wear resistance increases.
Normalization
Practice of heat treatment of steel
The following types of heat treatment are distinguished: annealing
,
normalization
,
hardening
and
tempering
of steel.
Annealing is the heating of steel above phase transformation temperatures, holding and subsequent slow cooling, usually with a furnace. After annealing, the steel approaches the phase and structural equilibria shown in the Fe–Fe3C diagram.
Therefore, after any annealing, the structure will be the same as in the iron-cementite diagram (see section 5.3):
· for hypoeutectoid steels – pearlite and ferrite;
· for eutectoid steel – pearlite;
· for hypereutectoid steels – pearlite and secondary cementite.
Depending on the heating temperature, annealing is divided into diffusion
,
complete
,
isothermal, recrystallization
and
incomplete
.
Diffusion annealing (homogenization) is heating the steel to a temperature of 1100...1200 ⁰C, holding it (minimum - about 16 hours), and then slowly cooling it in a furnace. It is used mainly for alloy steels and large shaped castings (ingots) made of carbon steel in order to equalize the chemical composition of carbon and alloying elements, and, consequently, the mechanical properties throughout the entire volume of the ingot (the higher the temperature, the higher the diffusion). After diffusion annealing, we obtain a structure consisting of pearlite and ferrite, but the grains will be large, which reduces the impact strength, and this is undesirable. Therefore, after diffusion annealing, it is necessary to refine the grain.
Methods of grinding grain. The grain in steel is crushed when heated at the moment when pearlite transforms into austenite. The cooling rate does not affect the grain size. Therefore, the grain in steel is crushed: 1) by complete annealing; 2) normalization; 3) hardening; 4) plastic deformation, in which grain refinement occurs mechanically, by crushing.
Full annealing is heating hypoeutectoid steel above Ac3 by 30...50 ⁰С (Fig. 6.1), holding and subsequent slow cooling with a furnace. It is impossible to heat it higher, since in this case the austenite grain grows too much. After such annealing, a fine-grained structure is obtained, consisting of pearlite and ferrite, with increased impact strength.
Rice. 6.1. Optimal heating temperature range
with various types of heat treatment
· for grain refinement and increasing impact strength after diffusion annealing, casting, welding, as well as after hot rolling and forging (1100...1200 ⁰С);
· to soften steel before cutting, since pearlite is much softer than martensite;
· to relieve internal stress.
Full annealing is usually a preparatory operation before the final heat treatment.
Full annealing for hypereutectoid steel, that is, heating above Acm , is not used, since: a) large grains are formed; b) a brittle network of secondary cementite appears along the grain boundaries.
Slow cooling in the furnace during complete annealing promotes the release of excess ferrite in the form of separate clusters, that is, grains. The formation of such sections (grains) of ferrite is undesirable, since during subsequent heating for hardening it is difficult to ensure equalization of the carbon concentration throughout the entire volume of austenite. This can lead to the formation of areas with reduced hardness after hardening. Therefore, rolled products, forgings, shaped castings, and alloy steel ingots are most often subjected to full annealing to reduce hardness in order to facilitate their rolling.
Isothermal annealing is a type of full annealing, used mainly for alloy steels in order to reduce annealing time.
And during complete and isothermal annealing, the parts are heated to temperatures above Ac3 by 30...50 ⁰C and a holding period is given to warm up the parts and complete the phase transformations of pearlite and ferrite in austenite. If full annealing is then carried out, then the parts are cooled in the same furnace. If isothermal annealing is carried out, then the parts from the first furnace are quickly transferred to the second furnace at a temperature corresponding to the isothermal holding. This results in a more solid sorbitol
or
troostite
, parts are processed worse by cutting, but there is a gain in time and less internal stress, since the pearlite transformation on the surface and in the core occurs more or less evenly, and there are no sharp stress concentrates.
Rolled products, stampings, tool blanks and other small-sized products are subjected to isothermal annealing. Heating is most often carried out in protective environments
to avoid oxidation and burning of carbon from the surface of parts. If, for example, the duration of conventional annealing of small-section high-speed steel is about 30 hours, then isothermal annealing lasts 8...10 hours.
When annealing very large products or workpieces, it is not possible to cool them quickly and uniformly in volume to the isothermal holding temperature. In this case, the transformation in individual parts of the parts occurs at different temperatures, which leads to an uneven structure and hardness.
Recrystallization annealing is the heating of cold-deformed steel above the recrystallization temperature, holding at this temperature and subsequent cooling. The purpose of annealing is to eliminate work hardening and increase ductility. This type of annealing is used before cold working and as an intermediate operation for removing cold hardening between cold working operations. In some cases, recrystallization annealing is also used as a final heat treatment.
The temperature of recrystallization annealing of steel depends on its composition. With an increase in the content of carbon and alloying elements in steel, the temperature of recrystallization annealing increases. Most often, the temperature of such annealing is in the range of 650...750 ⁰С (Fig. 6.1).
Incomplete annealing is heating hypoeutectoid and hypereutectoid steels above Ac1 by 30...50 ⁰C, holding and subsequent slow cooling with liver. The structure after such annealing is the same as in the iron-cementite diagram.
Hypoeutectoid steels. Incomplete annealing is used to soften steel before cutting, relieve internal stresses and partially refine the grain (the grain is refined only due to pearlite, and ferrite does not participate). This annealing is carried out when the hot pressure treatment is carried out correctly and does not lead to a sharp coarsening of the grain. Partial annealing is cheaper than full annealing and the steel is less likely to oxidize.
Hypereutectoid steels. Partial annealing is used instead of full annealing to refine the grain, since there is very little secondary cementite, and the structure contains mainly pearlite. In addition, partial annealing is used to obtain granular pearlite, and then this annealing is called cyclic or spheroidization
, since plastic cementite in perlite takes on a granular form.
The spheroidization process is as follows. When heated from 680...760 ⁰C and held, pearlite transforms into austenite during a eutectoid reaction. In this case, ferrite very quickly transforms into austenite (polymorphic transformation), and cementite then slowly dissolves in the resulting austenite, and the dissolution occurs mainly at the corners and edges of the plate, where it is easiest for atoms to come off.
When cooled from 760⁰C to 680⁰C and held, the reverse process occurs, that is, cementite falls out again, but mainly along the edges of the plate (rounding of the cementite plate in perlite). And this is repeated several times. As a result, we obtain granular pearlite, that is, against the background of ferrite there are not plates, but grains of cementite (Fig. 6.2).
Methods of hardening steels
In practice, various cooling methods are used depending on the size of the parts, their chemical composition and the required structure (diagram below).
Diagram: Cooling rates for different methods of hardening steels
Continuous hardening of steel
Continuous hardening (1) is a method of cooling parts in one environment. After heating, the part is placed in a quenching medium and left there until completely cooled. This technology is the most common and is widely used in mass production. Suitable for almost all types of structural steels.
Hardening in two environments
Quenching in two environments (speed 2 in the figure) is carried out in different quenching environments, with different temperatures. First, the part is cooled in the temperature range, for example, 890–400 °C, for example in water, and then transferred to another cooling medium - oil. In this case, the martensitic transformation will already occur in an oil environment, which will lead to a decrease in the leash and warping of steel. This hardening method is used for heat treatment of stamping tools. In practice, the opposite technological technique is often used - first the parts are cooled in oil and then in water. In this case, the martensitic transformation occurs in oil, and the parts are moved into water for faster cooling. This saves time on implementing the hardening technology.
Step hardening
During stepwise quenching (speed 3), the product is cooled in a quenching medium having a temperature higher than the martensitic transformation temperature. In this way, a certain isothermal holding is obtained before the transformation of austenite into martensite begins. This ensures uniform temperature distribution over the entire cross-section of the part. This is followed by final cooling, during which the martensitic transformation occurs. This method produces hardening with minimal internal stress. Isothermal holding can be done just below the temperature Mn, after the start of the martensitic transformation (speed 6). This method is more difficult from a technological point of view.
Isothermal hardening of steels
Isothermal hardening (speed 4) is done to obtain the bainitic structure of the steel. This structure is characterized by an excellent combination of strength and plastic properties. During isothermal hardening, parts are cooled in a bath of molten salts, which have a temperature 50–150 °C above the martensite point Mn, maintained at this temperature until the end of the transformation of austenite into bainite, and then cooled in air.
When hardening onto bainite, it is possible to obtain two different structures: upper and lower bainite. Upper bainite has a feathery structure. It is formed in the range of 500-350°C and consists of lath-shaped ferrite particles <1 µm thick and 5-10 µm wide, as well as thin cementite particles. The structure of upper bainite is characterized by higher hardness and strength, but lower ductility. Lower bainite has a needle-like martensite-like structure and is formed in the range of 350-200 °C. Lower bainite consists of fine particles of ε-carbides located in ferrite platelets. The bainite transformation never goes to completion. The structure always contains martensite and retained austenite. More preferable, in terms of performance characteristics, is the lower bainite structure. Products with such a structure are used in car construction, where parts experience shock-tensile stresses. The bainite hardening technology requires special hardening equipment. Additional materials on this technology can be found in the article “Technology of hardening for bainite.”
Cold treatment (5) is used for steels in which the temperature of the end of the martensitic transformation Mk is below room temperature.
High-speed steels, cemented parts, measuring instruments, and other particularly precise products are subjected to cold treatment. You can read more about this non-standard method of heat treatment in the article “Cold processing of steel parts”
The essence of processing
Normalization is heating a metal workpiece to a temperature 50 degrees above the critical temperature. After heating, cooling occurs. However, between these processes, exposure is carried out at a normalization temperature.
The degree of heating depends on the material of the part. To calculate the time of thermal exposure, it is necessary to pay attention to the homogenization of the metal structure. The optimal indicator is exposure for 1 hour at a thickness of 25 mm.
When cooling, certain points must be taken into account. When the temperature drops below critical, you need to speed up the cooling process. To do this, the part is dipped into a container with oil or water. The number of advantages and disadvantages of the finished product depends on correctly carried out heat treatment and subsequent cooling.
How to avoid scale formation and decarburization during quenching
Many steel parts are hardened after they have been finished. In such cases, it is unacceptable for the surface of the parts to be decarburized or for scale to form on it. There are methods for hardening steel products that avoid such problems. Hardening, carried out in a protective gas environment, which is injected into the cavity of the heating furnace, can be classified as the most advanced of these methods. It should be borne in mind that this method is used only if the heating oven is completely sealed.
The photo shows the moment of hydrobeating at the hot rolling mill - descaling
A simpler way to avoid decarburization of the metal surface during hardening is to use cast iron shavings and used carburizer. In order to protect the surface of the part when heated, it is placed in a special container into which these components are previously poured. To prevent ambient air from entering such a container, which can cause oxidation processes, the outside of it is thoroughly coated with clay.
If, after hardening the metal, it is cooled not in oil, but in a salt bath, it should be deoxidized regularly (at least twice per shift) to avoid decarburization of the surface of the part and the appearance of oxide on it. Boric acid, brown salt or charcoal can be used to deoxidize salt baths. The latter is usually placed in a special glass with a lid, the walls of which have many holes. Such a glass should be lowered into the salt bath very carefully, since at this moment a flame flares up on its surface, which dies out after a while.
There is a simple way to check the quality of deoxidation of a salt bath. To do this, a regular stainless steel blade is heated in such a bath for several minutes (3–5). After the salt bath, the blade is placed in water to cool. If after such a procedure the blade does not bend but breaks, then the deoxidation of the bath was successful.
Volumetric hardening of thick-walled workpieces
Steel normalization modes
This type of heat treatment implies:
- heating to temperatures of the austenitic state, which are slightly lower than the quenching temperature;
- short exposure at this temperature;
- air cooling.
Definition! The characteristics of normalized hot-rolled semi-finished products largely depend on the cross-section. The smaller the cross-sectional size, the shorter the cooling time and the higher the strength characteristics.
Differences between normalization and classical full annealing:
- cooling occurs not in the oven, but in air;
- cost-effectiveness, since normalization requires less time and money compared to annealing;
- ensuring complete recrystallization, which causes the appearance of a favorable fine-grained structure, higher strength, hardness and toughness.
Attention! As the carbon content increases, the difference between the characteristics of normalized and annealed steel increases. For grades containing up to 0.2% C, the more economical normalization is preferred. For medium- and high-carbon grades, the hardness of normalized steels is much higher than annealed ones, so in this case these two thermal operations are not always interchangeable.
Purpose
This technology is used to achieve the following goals:
- changes in the structure of an alloy or homogeneous metal;
- achieving greater strength and hardness;
- changes in the mechanical properties and characteristics of the part;
- reducing metal stresses that appear during other processing processes.
With the help of such thermal effects, you can achieve various results, for example, change the hardness and strength indicators.
Normalization is mandatory after processing steel by pressure, since increasing and decreasing the temperature allows you to correct problems with the structure of the material.
Possible defects during hardening
During the hardening process, some defects may appear in the workpieces. Only the most significant ones are described below.
Insufficient hardness
Insufficient hardness in a product that has undergone a hardening procedure most often appears when:
- the temperature of the heat treatment performed was incorrectly selected;
- the cooling rate was lower than that specified in the flow chart.
For example, during hardening of hypoeutectoid steels, this defect usually occurs due to the retention of ferrite in the structure of the alloy. This phenomenon occurs due to a violation of technology. In this case, the quenching temperature was simply not brought to the value corresponding to point Ac3.
Continuing the conversation about hypoeutectoid alloys, it is necessary to note another possible reason for the insufficient hardness of the material. This is overheating. As a result, martensite is formed, characterized by a coarse-needle structure. This structure not only reduces the hardness of the metal, but also reduces its impact strength. By the way, overheating manifests itself in a similar way in hypereutectoid steels.
Formation of soft spots
The reasons for the formation of soft spots are as follows:
- heterogeneity of the alloy structure;
- during the cooling process, the products came into contact with each other;
- uneven cooling;
- the presence of grease stains on the surface of the parts.
To correct this defect, the product is hardened again. Elimination of structural heterogeneity is carried out by preliminary normalization.
Carbon oxidation and combustion
Decarburization (the so-called burnout of carbon during hardening) and oxidation occur as a result of the interaction of the surface layer of the product with molten salts or furnace gases. The combination of these defects poses a particular danger to cutting tools. Its durability decreases significantly.
Such a defect in heat treatment cannot be corrected. The only thing that can save the situation is a sufficient allowance. Then the defective layers are removed by mechanical processing, and sometimes only grinding is sufficient.
Burnout
Burnout occurs when the heating temperature approaches the melting point of the metal. For this reason, what happens:
- penetration of oxygen into the thickness of the steel, accompanied by the formation of oxides at the grain boundaries;
- melting the material along the grain boundaries. This phenomenon, although rare, does happen.
As a result, the continuity of the alloy is broken, which puts it in the category of irreparable defect. That is, it is unsuitable for use.
Hardening cracks
The reasons for the appearance of hardening cracks are as follows:
- a part in the design of which there were sharp changes in the configuration of sections was subjected to heat treatment. It is in these places that significant internal stresses are formed, causing cracking;
- cooling was carried out extremely quickly;
- heating was carried out unevenly and also unnecessarily accelerated.
Another possible option for the appearance of cracks is that the product was subjected to a tempering procedure with some delay (not directly after hardening), due to which the internal stresses were not leveled in a timely manner.
Warping and deformation
Distortion of the product configuration - warping - causes uneven cooling. The change in volumetric characteristics - deformation - is associated with structural transformations that occur during heat treatment. These defects in the hardened alloy are due to the difference in the specific volumes of the formed structures. In particular, the value of this parameter of pearlite is less than that of martensite. In addition, thermal and structural stresses have different effects on the change in shape of different products.
To prevent the formation of these defects, the cooling procedure must be carried out at a slow rate in the temperature range of martensitic transformation using both isothermal and step hardening methods.
The essence of the steel normalization process
Most types of heat treatment of metal are carried out according to the same algorithm - heating, holding and cooling. These techniques allow you to change the structure and characteristics of the metal.
Heat treatment of metals
Despite the similarity of the process, each method has different time and temperature parameters. All types of influence on steel using temperature differences can use both an intermediate stage of the technical process and the final one.
The purpose of the intermediate stage is to prepare the steel for further processing, the final stage is to add new characteristics to the properties of the metal.
Normalization is used to minimize the number of grains in the metal structure formed by the weld. The temperature for this type of treatment is set based on the type of material.
So for alloys with a carbon content of 0.8-2.0% (hypereutectoid), a temperature regime with an interval between points Ac1 and Ac3 is used. For steel with a carbon content of up to 0.8% (hypoeutectoid) - more than Ac3.
As a result of normalization, materials of the first type acquire identical hardness, and the same amount of austenite is fixed. What emerges is a structure containing cement and martensite. This increases the hardness and wear resistance of the metal.
If a hypereutectoid metal is heated at temperatures above Ac3, its strength decreases, while the structure of a hypoeutectoid metal becomes more viscous.
The process time is determined by the standard index - 1 hour of exposure per 25 mm of metal thickness. Cooling depends on the size of the sheet and the amount of perlite.
There is a direct relationship between these quantities. So, with increasing cooling force, the thickness of the plates and the gap between them decreases, and the perlite becomes larger. Low cooling force leads to a decrease in the hardness and strength of the material.
Cooling Features
As is known, austenite is least stable at temperatures 550°С≤Т≤650°С. And the structure of martensite is formed when conditions are created for accelerated cooling of the alloy until its temperature index enters precisely this range. When the temperature falls below +240°C, the martensitic transformation is ensured by slow cooling. This technological solution leads to the fact that the stresses that arise in the metal body have time to level out. Moreover, without reducing the hardness of the formed martensite.
Successful heat treatment requires the correct choice of hardening medium. As such, the most commonly used are:
- mineral quenching oil;
- an aqueous solution of table salt (NaCl + H2O) or sodium hydroxide (NaOH);
- actually, water.
It is better to harden steel with alloying additives using oil. It is recommended to carry out this procedure with carbon alloys by cooling with water.
Features of the work
Normalization is uniform heating of the workpiece to a temperature above the critical one. After heating, the parts are maintained at the same temperature. Then the workpiece is cooled. Initially, it slowly cools down to the lower critical temperature, then the master immerses it in coolant to speed up the process.
Principles of conducting
Heat treatment of metal is necessary if its structure and, consequently, technical characteristics change.
There are two types of metals that can be heat treated:
The choice of temperature depends on the type of metal. For example, for hypereutectoid billets, the heating process is carried out at temperatures located between the marks AC1–AC3. As for hypoevectoid parts, they are processed at temperatures above the AC3 point. Materials belonging to the first group achieve the same hardness index.
Duration
The cooling rate depends on the amount of perlite contained in the workpiece and the size of the plates being processed. If the cooling rate is increased and the procedure time is reduced, the amount of perlite formed during the heat treatment process will increase. Strength and hardness indicators will also increase.
Cooling steel
Annealing of hypereutectoid steel.
For hypereutectoid steels
I most often use partial annealing
, which is also called
spheroidization
, since this is the main method for producing granular perlite. It consists of heating hypereutectoid steel to temperatures not much higher (10 - 300 C) than the critical point Ac1, holding it and then slowly cooling it. After heating in the Ac1 – Ac3 range, a large number of undissolved cementite inclusions remain in the austenite, which serve as crystallization centers during the decomposition of austenite during cooling. As a result, a structure of granular perlite is formed (Fig. 4, b). Steel with a granular pearlite structure has lower hardness and strength, and, accordingly, higher ductility; it is easier to machine. In addition, granular pearlite is the optimal initial structure before hardening. Increasing the annealing temperature to higher values leads, upon subsequent cooling, to the formation of lamellar pearlite and, as a consequence, to an increase in hardness. Tool and bearing steels are processed into granular perlite.
for hypereutectoid steels with heating above Acm (line ES) they are not used at all, since with slow cooling after such heating a coarse network of secondary cementite is formed along the grain boundaries, which worsens the mechanical properties of the steel.
Normalization
Normalization is a type of heat treatment in which steel is heated above critical points, followed by cooling to 20 0C in still air (V2, see Fig. 2). Cooling in air leads to the decomposition of austenite at greater undercooling than during annealing. Therefore, during normalization, a finer eutectoid structure and a smaller eutectoid grain are obtained, which causes an increase in the strength of the steel. Normalization is a shorter and cheaper operation than annealing, because The thermal furnace is not used for cooling.
Purpose of normalization. Normalization of hot-rolled steel refines the grain and increases its resistance to brittle fracture, which is characterized by a decrease in the cold brittleness threshold and an increase in the work of crack development.
Selection of normalization temperatures. Normalization consists of heating hypoeutectoid steel to a temperature exceeding the Ac3 point by 30-500 C, and hypereutectoid steel above Acm also by 30-500 C (Fig. 3), holding for a short time to warm up the charge and complete phase transformations, and cooling in air. Normalization causes complete phase recrystallization of the steel and eliminates the coarse grain structure obtained during casting or rolling, forging or stamping. In hypereutectoid steels, accelerated cooling in air in the intercritical interval (Acm - Ac1) prevents the precipitation of a network of secondary cementite around austenite grains, and, consequently, a decrease in the properties of the alloy.
Accelerated cooling in air leads to the decomposition of austenite at lower temperatures than during annealing, which increases the dispersion of the ferrite-cementite structure. After normalization, a fine ferrite-cementite mixture is formed, called sorbitol.
The cooling rate during normalization is in the range of 20 – 500C/min.
Normalization increases the strength and hardness of medium- and high-carbon steel by 10–15% compared to annealed steel.
When steel is cooled in an air flow (V3, see Fig. 2), where the cooling rate reaches 800 C/sec, the most dispersed ferrite-cementite mixture is formed - troostite. The more dispersed the resulting structure, the higher its strength and hardness (perlite has a hardness of HB 180¸230
, sorbitol – HB 250¸350
, troostite –
HB 350 ¸ 500
). Ferrite-cementite structures (pearlite, sorbite, troostite) obtained by direct decomposition of supercooled austenite have a lamellar structure.
Hardening is the heating of steel above the critical point followed by rapid cooling. The cooling rate during quenching must be high enough (above Vcr, see Fig. 2) so that diffusion decomposition of austenite does not occur when the temperature decreases. Quenching is not the final heat treatment operation. To obtain the required mechanical properties, steel after hardening is most often tempered.
Purpose of hardening. Structural steel is subjected to quenching and tempering to increase strength, hardness, toughness, and for a number of parts also for high wear resistance. Tool steel is hardened to increase hardness, strength, and wear resistance.
Choice of hardening temperatures. For hypoeutectoid steels, the heating temperature for hardening is taken to be 30–500 C above Ac3, and for hypereutectoid steels 30–500 C above Ac1 (Fig. 5). After holding the steel, rapid cooling is carried out (30 ¸ 2000 C/sec) to temperatures at which there is no diffusion decomposition (V4, see Fig. 2). The following coolants are used for hardening: water, oils (mineral), molten salts and molten metals. As a result of quenching, austenite transforms into martensite. Martensite is a supersaturated solid solution of carbon in a-iron. Martensite is characterized by a needle-like, packet or plate-like structure (Fig. 6), high hardness ( HB 600 ¸ 700
), high fragility and the presence of high internal stresses.
Figure 5. Section of the iron-carbon diagram with the optimal heating temperature range for hardening carbon steels
Figure 6. Microstructure of martensite
Heating hypoeutectoid steel above Ac1 but below Ac3 retains ferrite in the hardened steel, which reduces the hardness in the hardened state and worsens its mechanical properties after tempering. Therefore, incomplete hardening
, i.e. Heating above Ac1 but below Ac3 is usually not used for hypoeutectoid steels.
For hypereutectoid steels, on the contrary, the optimal quenching temperature lies in the range Ac1 – Acm, i.e. incomplete hardening is optimal. The presence of excess cementite in the structure of hardened steel is beneficial in many ways; for example, inclusions of excess cementite increase the wear resistance of steel. Heating above Ac3 is dangerous and unnecessary, since it does not lead to an increase in hardness (the hardness even drops somewhat due to the dissolution of excess cementite and an increase in the amount of retained austenite). In addition, austenite grains grow, the possibility of large quenching stresses increases, and the steel is more intensively decarburized from the surface.
Steel hardening
Hardening of steel Hardening is a heat treatment operation consisting of heating to temperatures above the upper critical point AC3 for hypoeutectoid steel and above the lower critical point AC1 for hypereutectoid steel and holding at this temperature followed by rapid cooling (in water, oil, aqueous solutions salts, etc.). As a result of hardening, steel acquires a martensite structure and, as a result, becomes hard. Hardening increases the strength of structural steels and imparts hardness and wear resistance to tool steels. Hardening modes are determined by the heating rate and temperature, the duration of exposure at this temperature, and especially the cooling rate. Choice of hardening temperature. The heating temperature of steel for hardening depends mainly on the chemical composition of the steel. When hardening hypoeutectoid steels, heating should be carried out to a temperature 30 - 50° above the AC3 point. In this case, the steel has the structure of homogeneous austenite, which, upon subsequent cooling at a rate exceeding the critical hardening rate, turns into martensite. This type of hardening is called complete. When hypoeutectoid steel is heated to temperatures AC1 - AC3, a certain amount of ferrite remaining after hardening is retained in the martensite structure, which reduces the hardness of the hardened steel. This type of hardening is called incomplete.
For hypereutectoid steel, the best quenching temperature is 20-30° above AC1, i.e. incomplete quenching. In this case, the preservation of cementite during heating and cooling will contribute to an increase in hardness, since the hardness of cementite is greater than the hardness of martensite. Hypereutectoid steel should not be heated to a temperature above AC1, since the hardness is lower than when quenched from a temperature above AC1, due to the dissolution of cementite and an increase in the amount of retained austenite. In addition, when cooling from higher temperatures, large internal stresses can arise.
Cooling rate. To obtain the martensite structure, it is necessary to supercool the austenite by rapidly cooling the steel, which is at the temperature of the lowest stability of austenite, i.e., at 650-550 ° C. In the temperature zone of martensitic transformation, i.e., below 240 ° C, on the contrary , it is more profitable to use slow cooling, since the resulting structural stresses have time to level out, and the hardness of the resulting martensite practically does not decrease. The correct choice of quenching medium is of great importance for successful heat treatment. The most common quenching media are water, a 5-10% aqueous solution of caustic soda or table salt, and mineral oil. For hardening carbon steels, water with a temperature of 18° C can be recommended; and for hardening most alloy steels - oil.
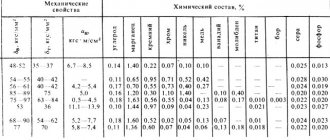
Hardenability and hardenability of steel. When hardening steel, it is important to know its hardenability and hardenability. These characteristics should not be mixed.
Hardenability shows the ability of steel to increase hardness when hardened. Some steels have poor hardenability, i.e. have insufficient hardness after hardening. Such steels are said to “not accept” hardening. The hardenability of steel depends mainly on its carbon content. This is explained by the fact that the hardness of martensite depends on the degree of distortion of its crystal lattice. The less carbon there is in martensite, the less distorted its crystal lattice will be and, consequently, the lower the hardness of the steel will be. Steels containing less than 0.3% carbon have low hardenability and therefore, as a rule, are not subjected to hardening.
The hardenability of steel is characterized by its ability to be hardened to a certain depth. During hardening, the surface of the part cools faster, since it is in direct contact with the coolant, which removes heat. The core of the part cools much more slowly; heat from the central part of the part is transferred through the mass of metal to the surface and is absorbed by the coolant only at the surface. The hardenability of steel depends on the critical hardening speed: the lower the critical speed, the greater the depth the steel parts are hardened. For example, steel with a large natural grain of austenite (coarse-grained), which has a low critical hardening rate, is annealed to a greater depth than steel with a small natural grain of austenite (fine-grained), which has a high critical hardening rate. Therefore, coarse-grained steel is used for the manufacture of parts that must have deep or through hardenability, and fine-grained steel is used for parts with a hard surface hardened crust and a viscous unhardened core. The depth of hardenability is also influenced by the initial structure of the steel being hardened, the heating temperature for hardening and the quenching medium. The hardenability of steel can be determined by fracture, microstructure and hardness.
Types of steel hardening. There are several hardening methods used depending on the composition of the steel, the nature of the workpiece, the hardness that needs to be obtained, and the cooling conditions. Quenching in one environment is shown schematically in Fig. 1 in the form of curve 1. Such hardening is easier to perform, but it cannot be used for every steel and not for all parts, since rapid cooling of parts with variable cross-sections in a large temperature range contributes to the occurrence of temperature unevenness and large internal stresses, which can cause warping of the part, and sometimes cracking (if the magnitude of internal stresses exceeds the tensile strength). The more carbon in steel, the greater the volumetric changes and structural stresses, the greater the risk of cracks.
Rice. 1. Cooling curves for various hardening methods
Hypereutectoid steels are hardened in one environment if the parts have a simple shape (balls, rollers, etc.). If the parts have a complex shape, either hardening in two environments or step hardening is used. Hardening in two environments (curve 2) is used for tools made of high-carbon steel (taps, dies, cutters). The essence of the method is that the part is first soaked in water, quickly cooling it to 300-400 ° C, and then transferred to oil, where it is left until completely cooled.
Step hardening (curve 3) is performed by rapidly cooling the parts in a salt bath, the temperature of which is much higher than the temperature at which the martensitic transformation begins (240-250 ° C). Holding at this temperature should ensure equalization of temperatures across the entire cross-section of the part. The parts are then cooled to room temperature in oil or in still air, thereby eliminating thermal internal stresses. Step hardening reduces internal stresses, warping and the possibility of cracking. The disadvantage of this type of hardening is that hot traces cannot provide a high cooling rate at a temperature of 400-600 ° C. In this regard, step hardening can be used for parts made of carbon steel with a small cross-section (up to 8-10 mm). For alloy steels that have a low critical hardening rate, step hardening is applicable to parts with a large cross-section (up to 30 mm).
Isothermal hardening (curve 4) is carried out in the same way as step hardening, but with a longer exposure at a hot bath temperature (250-300 ° C) to ensure complete decomposition of austenite. The holding time required for complete decomposition of austenite is determined by points a and b and by the S-shaped curve (see Fig. 1). As a result of such hardening, the steel acquires the structure of acicular troostite with a hardness of HRC45 55 and maintaining the necessary ductility. After isothermal hardening, steel can be cooled at any speed. Molten salts are used as a cooling medium: 55% KNO3 + 45% NaNO2 (melting point 137 ° C) and 55% KNO3 + 45% NaNO3 (melting point 218 ° C), allowing overheating to the required temperature. Isothermal hardening has the following advantages over conventional hardening: minimal warping of steel and absence of cracks; high viscosity of steel. Currently, stepwise and isothermal light hardening are widely used.
Light hardening of steel parts is carried out in specially equipped furnaces with a protective environment. In some tool factories, to obtain a clean and bright surface of a hardened tool, step-by-step hardening with cooling in molten caustic alkali is used. Before hardening, the tool is heated in a salt bath of sodium chloride at a temperature 30-50 ° C above point AC1 and cooled at 180-200 ° C in a bath consisting of a mixture of 75% caustic potassium and 25% caustic soda with the addition of 6 -8% water (by weight of all salt). The mixture has a melting point of about 145° C and, due to the fact that it contains water, has a very high hardening ability.
During stepwise hardening of steel with supercooling of austenite in molten caustic alkali, followed by final cooling in air, the parts acquire a clean, light, silver-white surface; in this case, there is no need for sandblasting the parts and washing them in hot water is sufficient.
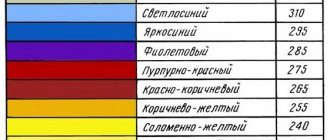
Self-tempering hardening is widely used in tool production. Its essence lies in the fact that the parts are not kept in the cooling medium until completely cooled, but at a certain moment they are removed from it in order to retain a certain amount of heat in the core of the product, due to which subsequent tempering is carried out. After reaching the required tempering temperature due to internal heat, the part is finally cooled in the quenching liquid. You can control the tempering by the tarnish colors (see Fig. 2) that appear on the cleaned steel surface at 220–330° C.
Rice. 2. Tempering colors
Self-tempering hardening is used for chisels, sledgehammers, bench hammers, punches and other tools that require high surface hardness and preservation of a viscous core.
Methods of cooling during hardening. Rapid cooling of steel parts during hardening causes the occurrence of large internal stresses in them. These stresses sometimes lead to warping of parts, and in the most severe cases, cracks. Particularly large and dangerous internal stresses arise during cooling in water. Therefore, where possible, parts should be cooled in oil. However, in most cases this is not possible for carbon steel parts, since the cooling rate in oil is much less than the critical speed required for the transformation of austenite into martensite. Consequently, it is recommended to harden many parts made of carbon steels with cooling in water, but at the same time reduce the inevitably occurring internal stresses. To do this, they use some of the described hardening methods, in particular, hardening in two environments, hardening with self-tempering, etc. Internal stresses also depend on the method of immersing parts in the hardening medium. It is necessary to adhere to the following basic rules: parts with thick and thin parts should be immersed in the quenching medium with the thick part first; parts that have a long elongated shape (taps, reamer drills) should be immersed in a strictly vertical position, otherwise they will warp (Fig. 3).
Rice. 3. Correct immersion of parts and tools in the hardening medium
Sometimes, due to operating conditions, not the entire part must be hardened, but only part of it. In this case, local hardening is used: the part is not completely heated, but is completely immersed in the hardening medium. In this case, only the heated part of the part is hardened. Local heating of small parts is carried out in a salt bath, immersing only that part of the part that needs to be hardened; This is how, for example, the centers of lathes are hardened. You can also do this: heat the part completely, and cool in the quenching environment only the part that needs to be quenched.
Defects that occur during hardening of steel. Insufficient hardness of the hardened part is a consequence of low heating temperature, short exposure at operating temperature or insufficient cooling rate. Defect correction: normalization or annealing followed by hardening; use of a more energetic quenching medium.
Overheating is associated with heating the product to a temperature significantly higher than the required heating temperature for hardening. Overheating is accompanied by the formation of a coarse-grained structure, resulting in increased brittleness of the steel. And correction of the defect: annealing (normalization) and subsequent hardening at the required temperature.
Burnout occurs when steel is heated to very high temperatures, close to the melting point (1200-1300 ° C) in an oxidizing atmosphere. Oxygen penetrates into the steel, and oxides form along the grain boundaries. Such steel is brittle and cannot be repaired. Oxidation and decarbonization of steel are characterized by the formation of scale (oxides) on the surface of parts and burnout of carbon in the surface layers. This type of defect cannot be corrected by heat treatment. If machining allowance allows, the oxidized and decarburized layer must be removed by grinding. To prevent this type of defect, it is recommended to heat the parts in ovens with a protective atmosphere. Warping and cracks are consequences of internal stresses. During heating and cooling of steel, volumetric changes are observed, depending on temperature and structural transformations (the transition of austenite to martensite is accompanied by an increase in volume up to 3%). The different times of transformation in the volume of the hardened part due to its different sizes and cooling rates across the cross section lead to the development of strong internal stresses, which cause cracks and warping of parts during the hardening process. The formation of cracks is usually observed at temperatures below 75-100 ° C, when the martensitic transformation covers a significant part of the steel volume. To prevent the formation of cracks, when designing parts, it is necessary to avoid sharp protrusions, pointed corners, and sharp transitions from thin to thick sections; The steel should also be slowly cooled in the zone of martensite formation (quenching in oil, in two environments, step quenching). Cracks are an irreparable defect, but warping can be eliminated by subsequent straightening or straightening.
The main transformations in iron-carbon alloys during slow heating and cooling Line on the diagram Transformation temperature, °C Description of transformation Designation of critical points PSK 723 Transformation of pearlite into austenite. Transformation of austenite into pearlite Ac1, Ar1 MO 768 Loss of magnetic properties for steels with a carbon content of up to 0.5%. The emergence of magnetic properties for the same steels. Ac2, Ar2 GS 723-910 Completion of dissolution of ferrite in austenite in hypoeutectoid steels. Beginning of ferrite precipitation from austenite in hypoeutectoid steels. Ac3, Ar3 SE 723-1130 Completion of dissolution of cementite in austenite in hypereutectoid steels. The beginning of the separation of cementite from austenite in hypereutectoid steels. Acm, Arm IE - The beginning of steel melting when heated. The end of steel solidification during cooling - ECF - The beginning of cast iron melting during heating. The end of cast iron solidification during cooling -
Source: Ostapenko N.N., Krapivnitsky N.N. Metal technology. M. Higher school, 1970
Other heat treatment methods
In addition to the normalization process, there are other methods of heat treatment of metals and alloys:
- Tempering is a technology used to reduce brittleness and internal stresses in a material.
- Annealing is a method in which the grain size in the structure of a material is reduced and internal stress is relieved.
- Hardening is a technique similar to normalization. The differences are a higher heating temperature and a high cooling rate.
- Cryogenic processing is a technology associated with the use of low temperatures.
- Dispersion hardening is the final stage of heat treatment. The processed part is given a high strength index.
The main methods for processing metal workpieces are presented above, but the order is incorrect. You can find it out in any source on metalworking.
Steel normalization is considered one of several stages of heat treatment. With its help, the structure and characteristics of the material change. If desired, you can worsen or improve the properties of the workpiece.
Features of hardening of various types of steel - methods, temperature, other nuances
One of the most common methods of heat treatment of metals is steel hardening. It is with the help of hardening that the required characteristics of the finished product are formed, and its incorrect implementation can lead to excessive softness of the metal (non-hardening) or to its excessive fragility (overheating). Our article will talk about what proper hardening is and what needs to be done to achieve it.
Digital library
General technical disciplines / Technological equipment / 3.2 Normalization and standardization of elements, components and designs of devices
The direction of normalization and standardization of elements, components and designs of devices is due to frequent changes in products and the desire to leave the device unchanged. This contradiction is resolved by normalization, unification and standardization of parts and assembly units of devices. At the same time, the volume of design work is reduced, the nomenclature is reduced, and the number of parts to be manufactured of the same name and size increases.
Normalized or standard parts can be produced in large quantities in a centralized manner, which reduces their cost. Normalized and standard parts and assembly units can be removed from used devices and, after partial repairs (if necessary), transferred to the warehouse. They can be reused when assembling new fixtures.
In the field of devices, standardization covers:
· structural and dimensional elements of parts (threads, cones, key connections, slopes, fits, etc.);
· assembly units of devices;
· some designs of devices;
· elements of power drives;
The standardization carried out in the field of equipment makes it possible to design devices using standard parts and assembly units in the amount of 30 to 90% of the total number of parts in the design.
Normalization (ordering) includes the following stages:
1) normalization of general structural and dimensional elements. The object of normalization is:
— size ranges for elements of devices;
— overall and connecting dimensions;
— structural elements (threads, fastening parts, pins, key connections, slopes, etc.;
— fits for the applied mates and tolerances for the main parts;
2) normalization of parts of special devices (installation elements, parts of clamping devices, bodies of devices and their elements, installations for checking the position of tools, parts of auxiliary devices), as well as their blanks (castings, forgings);
3) normalization of assembly units of devices for various functional purposes (pneumatic and hydraulic cylinders, pneumatic chambers, dividing and rotating mechanisms, clamps, ejectors, etc.).
Cooling methods
When hardening steel parts, which is carried out with accelerated cooling, the likelihood of significant internal stresses occurring is very high. For this reason, metal warping occurs. Even cracking is possible. Preventing these negative phenomena is possible by cooling products in an oil environment, of course, if their production technology allows this.
A different approach is relevant for carbon steels. They cannot be cooled in oil. Therefore, this operation must be performed in water.
In addition to the cooling medium, the method of immersing workpieces into it is important from the point of view of the formation of internal stresses. In this case, the following rules should be followed:
- It is necessary to immerse parts, the design of which includes thin and thick fragments, into the hardening substance, starting with the larger one;
- drills, tools with which internal threads are cut - taps - in general, products characterized by an elongated configuration should be immersed without allowing their longitudinal axis to deviate from the vertical. Then they won't warp.
There are cases when it is necessary to harden only part of the part. This problem is solved by using local heat treatment. Only the required fragment of the product is heated, and the entire part is subject to immersion in the quenching liquid.
Recrystallization annealing
A technique that allows you to get rid of many undesirable qualities of metal. Recrystallization annealing of steel is carried out in order to remove cold hardening and other consequences after certain mechanical operations. The technology is used to process:
After recrystallization annealing of steel, the metal acquires the necessary characteristics to obtain products with the specified qualities.
The choice of technology is determined by the chemical composition. During the procedure, the material is heated to values exceeding the crystallization temperature by at least 100-200 ° C. The necessary properties appear to varying degrees depending on the type of treatment. More often, full annealing is used. At the same time, structural changes are more significant. In some cases, incomplete annealing is sufficient.
Temperature zones for recrystallization annealing
Steel after hardening: structure and properties
Steel in its usual form is a fairly soft and malleable metal. Some grades do not require special strength (these are the so-called ordinary quality steels, produced in accordance with the requirements of GOST 380): the indicators obtained after smelting are quite sufficient, for example, for sewer manholes or protective gratings. But there are categories of steels - structural and instrumental - for which the initial strength indicators are not enough. They must be subjected to heat treatment. Its main type is hardening.
Microstructure of steel 45 after annealing and hardening
Hardening: the essence of the operation
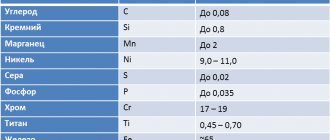
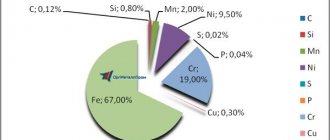
As is known, any steel is a solid solution of carbon in the main structure of α-iron. In this case, the grade determines the percentage of carbon content (for example, the grade “65 steel” means that it contains 0.65% C, U13 steel contains about 1.3% C, and so on). However, this element is quite chemically active, therefore, during the smelting process (at 1600...2000 °C) it is actively bound by iron, resulting in the formation of Fe3C cementite. Everything else is ferrite - a fairly soft structural component. The large amount of ferrite in low-carbon steels causes their increased ductility, even in a cold state. This does not apply to steels:
- alloyed (they are produced according to the requirements of GOST 4543);
- bearings according to GOST 801;
- spring-spring according to GOST 2052 and GOST 14959;
- all types of instrumental, both alloyed and unalloyed.
To understand the effectiveness of hardening, it is necessary to refer to the structure of the steel after smelting and subsequent hot rolling into the required profile - strip, rod or special profile (angle, channel, etc.).
Any steel has a crystalline structure, which is made up of an infinite number of crystals. If steel is poured and the melt is then cooled, these crystals turn into multifaceted formations called grains. Since active saturation with oxygen occurs, voids appear between adjacent crystals, which, during the cooling of the ingot, are gradually filled with sulfur, phosphorus and other low-melting non-metallic inclusions. This not only reduces ductility (phosphorus and sulfur are very fragile chemical elements), but also contributes to the appearance of very coarse accumulations of grains, which makes the metal uneven in its density. It is impossible to process such products - the ingot will begin to split. Therefore, immediately after smelting, rolling is performed, during which the original defects are healed and the structure becomes more homogeneous. Accordingly, the density increases and surface cracks disappear.
Temperature of the workpiece depending on the color when heated
Plastic deformation has a positive effect only on the macrostructure. Hardening is responsible for changing the microstructure - a set of technological methods of heat treatment, the essence of which is to increase the strength characteristics of steel. The point of hardening is to fix a number of high-temperature components of the microstructure (giving steel resistance) for normal operating conditions of products. Accordingly, steel, without changing its chemical composition, will sharply increase the level of some of its mechanical characteristics:
- limit of temporary resistance σв, MPa;
- yield strength σt, MPa;
- fatigue limit σi, MPa;
- hardness according to Brinell HB or Rockwell HRC.
At the same time, some indicators - in particular, impact strength, relative elongation - become lower after hardening. If this is critical from the point of view of the subsequent operational durability of the part (and in most cases this is the case), then it is correct to perform a number of additional operations after hardening: tempering, aging, etc.
Annealing steel
The range of metal products is huge and in each case certain, often specific qualities of the material are required. The manufacturer is not able to provide a complete list of brands. Metallurgical enterprises offer raw materials that meet GOST, which are subsequently refined in manufacturing plants. One of the key operations is steel annealing. At this stage, the metal acquires the necessary technical properties for subsequent processing. To understand what steel annealing is, you need to understand why it is done and what processes occur during this process.
What is metal annealing
Annealing of metal is used to obtain an equilibrium and homogeneous structure when preparing a product for subsequent thermal or mechanical treatment, as well as to improve its physical characteristics after cutting, welding, stamping, rolling or hardening operations. The purpose of annealing is to eliminate internal inhomogeneities of steel, improve its grain size and uniformity of the crystal lattice, and also remove residual stress caused by deformation of the product during various types of processing. Features of this technology allow:
- bring the properties of steel to the requirements of subsequent heat treatment;
- improve the characteristics of the workpiece material before cutting or pressure processing;
- prevent deformation and eliminate internal stresses of welded and cast products;
- restore the original quality of steel after unsuccessful hardening.
One of the characteristic features of this heat treatment is that the heated metal cools naturally, without the use of cooling media. And the heating temperature during annealing depends on the composition of the steel and the desired result.
What is the hardening of metal?
The ancient blacksmiths knew that the effect of high temperature on metal can change its structure and properties and actively used this in practice. Subsequently, it was scientifically established that hardening of products made of steel, which involves heating and subsequent cooling of the metal, can significantly improve the mechanical characteristics of finished products, significantly increase their service life and even ultimately reduce their weight by increasing the strength of the part. What’s noteworthy is that hardening parts made from inexpensive steel makes it possible to give them the required characteristics and successfully use them instead of more expensive alloys.
The meaning of the process, which is called hardening of steel alloy products, is to heat the metal to a critical temperature and then cool it. The main goal pursued by this heat treatment technology is to increase the hardness and strength of the metal while simultaneously reducing its ductility.
There are various types of hardening and subsequent tempering, differing in modes of implementation, which determine the final result. Hardening modes include the heating temperature, the time and speed of its implementation, the time the part is kept in a state heated to a given temperature, and the speed at which cooling is carried out.
The most important parameter when hardening metals is the heating temperature, upon reaching which the atomic lattice is rearranged. Naturally, for different grades of steel, the critical temperature value is different, which depends, first of all, on the level of carbon content and various impurities in their composition.
After hardening, both the hardness and brittleness of the steel increases, and a layer of scale appears on its surface, which has lost a significant amount of carbon. The thickness of this layer must be taken into account when calculating the allowance for further processing of the part.