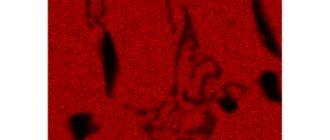
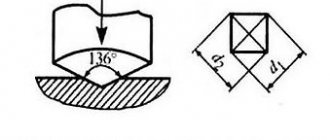
When conducting metal examination, the main types of structural analysis are metallographic studies of metals .
Metallographic research is a set of tests and analytical activities aimed at studying the macrostructure and microstructure of metals, studying the patterns of structure formation and the dependence of the influence of the structure on the mechanical, electrical and other properties of the metal (alloy). Analysis of the structure of metals and alloys is an integral part of metallurgical expertise. It allows you to answer many questions regarding the detection of internal defects in metal, unacceptable structural components, traces of heat treatment and plastic deformation, as well as the presence or absence of surface layers. Information on all of these factors forms an overall picture of the quality of the material being studied.
Materials science and heat treatment of steels. Methods for studying the structure of metals and alloys
It is customary to distinguish the structure of metals and alloys into: macrostructure, microstructure and fine structure. Depending on the structure of metals and alloys, there are three methods for studying them:
- Macroscopic analysis
- Microscopic analysis
- X-ray structural analysis and X-ray flaw detection
Macroscopic analysis.
Macrostructure is the structure of metals and alloys, which is visible to the naked eye or at low magnifications with a magnifying glass (max. 30 times). Macrostructure is studied through macroanalysis.
Metals are opaque substances and their structure is studied in fractures or specially prepared samples (macrosections). The sample is cut from a certain place, in a certain plane, depending on what is being examined (casting, forging, stamping, rolling, welded or heat-treated part) and what needs to be identified and studied (primary crystallization, heterogeneity of structure, defects that violate the continuity of the metal ). Therefore, samples are cut from one or more places in the ingot (or blank, or part) in both the longitudinal and transverse directions. The surface of the sample (template) is leveled on an emery wheel and then ground. After grinding, the template is etched in special reagents, which dissolve structural components in different ways and etch defects.
Macroanalysis reveals:
- type of fracture (brittle, tough);
- size, shape and location of grains and dendrites of cast metal;
- defects in ingots and castings (shrinkage cavities, gas bubbles, cracks);
- defects that disrupt the continuity of the metal (shrinkage porosity, gas bubbles, cavities, cracks);
- chemical heterogeneity of the metal caused by crystallization processes or created by thermal and chemical-thermal treatment;
- arrangement of fibers in forged and stamped workpieces;
- cracks that occur during pressure treatment or heat treatment, defects in welds.
Microscopic analysis
A more subtle method of studying the structure and defects of metals is microanalysis, i.e. studying the structure of metals at high magnifications using a metallographic microscope.
Microscopic analysis is the study of a surface using light microscopes, where magnification within the range of 50...2000 times allows one to detect structural elements up to 0.2 microns in size.
Metallographic microscopes.
A metallographic microscope examines metal in reflected light (the main difference from a biological microscope, where an object is viewed in transmitted light). Much higher magnification can be obtained using an electron microscope, in which rays of light are replaced by a stream of electrons (up to 100,000 times magnification is achieved).
Transmission microscopes.
A flow of electrons passes through the object being studied. The image is the result of unequal scattering of electrons by an object. There are indirect and direct research methods.
With the indirect method, it is not the object itself that is studied, but its imprint - a quartz or carbon cast (replica), displaying the microsection relief, to prevent secondary radiation, which distorts the picture.
With the direct method, thin metal foils up to 300 nm thick are studied through transmission. Foils are obtained directly from the metal being studied.
Scanning microscopes.
The image is created due to the secondary emission of electrons emitted by the surface onto which a stream of primary electrons continuously moving along this surface falls. The surface of the metal is studied directly. The resolution is slightly lower than that of transmission microscopes.
To study the microstructure, thin sections (microsections) are also prepared. Here, after grinding, additional polishing is carried out to a mirror finish, then the section is etched.
Microanalysis allows you to identify:
- size, shape and location of grains;
- individual structural components of the alloy, on the basis of which the chemical composition of annealed carbon steels can be determined;
- quality of heat treatment (for example, depth of hardening penetration);
- various defects (burnout, decarburization, presence of non-metallic inclusions).
X-ray structural analysis and X-ray flaw detection
X-rays have the same nature as light rays and are electromagnetic vibrations, with a wavelength from 2 x 10-7 to 10-9 cm (the length of light rays from 7.5 x 10-5 to 4 x 10-5 cm).
X-rays are produced in X-ray tubes as a result of the deceleration of electrons when they collide with the surface of a metal. In this case, the kinetic energy of electrons is converted into the energy of x-rays.
X-ray diffraction analysis is based on the ability of atoms to reflect X-rays in a crystal lattice. The reflected rays leave a group of spots or rings on the photographic plate (x-ray). The nature of their arrangement determines the type of crystal lattice, as well as the distance between atoms (positive ions) in the lattice.
X-ray scanning is based on the ability of X-rays to penetrate deep into the body. Thanks to this, it is possible, without cutting metal products, to see on an x-ray image various internal metal defects (shrinkage cavities, cracks, welding defects).
Methods for recording defects in a material are based on the fact that X-rays are partially absorbed when passing through the metal. At the same time, less dense parts of a metal product (areas with defects) absorb rays less than dense parts (solid metal). This leads to the fact that on an x-ray image, areas with defects will have dark or light spots against the background of solid metal.
Modern X-ray machines make it possible to examine steel products to a depth of 60–100 mm.
Gamma rays are used to detect defects in thick metal products. Gamma rays are similar in nature to X-rays, but their wavelengths are shorter. Due to the high penetrating power of gamma rays, they can illuminate steel parts up to 300 mm thick.
Metallography Laboratory
If you're thinking about how to determine the structure of steel or other metal, the answer is simple - optical microscopy. It allows you to use magnifications up to 2000x. The disadvantage of this research method is that it requires high-quality sample preparation - the production of microsections.
Sample preparation is a key step in the work of metallography laboratory staff. Sample preparation is divided into two stages. The first stage of sample preparation is obtaining a representative sample of a certain size, weight and composition. The second stage is bringing the sample into the state required to determine the structure of the metal (for example, grinding, polishing and etching for microstructure).
After obtaining a thin section, they proceed to direct study of the structure. To do this, the required magnification, illumination mode, additional optical prisms and filters are selected on the microscope.
Assay method
Assay method: Assay melting is based on the physical and chemical laws of metal reduction, slag formation and wetting with molten substances. The main stages of assay analysis using the example of an alloy of silver and lead:
- Sample preparation
- Mixing
- Crucible melting for lead alloy
- Pouring lead alloy into iron molds for cooling
- Separation of lead alloy (werkbley) from slag
- Werkbley cupellation (lead removal)
- Removing a bead of precious metals and weighing it
- Quartering (adding silver, if necessary)
- Treating the bead with dilute nitric acid (dissolving silver)
- Gravimetric (weight) determination of silver
X-ray fluorescence analysis
XRF analysis is carried out by exposing the metal to X-rays and analyzing the fluorescence using modern electronics to achieve good measurement accuracy.
Advantages of the method:
- Non-destructive analysis.
- It is possible to measure many elements with high accuracy.
Alloy identification is achieved by identifying the unique combination of several elements within specified compositional ranges. Accurate quantitative analysis is achieved by using appropriate corrections to the inter-element influence matrix.
The analyzed material is exposed to X-ray fluorescence within a few seconds. Atoms of elements in a material become excited and emit photons with energies specific to each element. The sensor separates and stores photoelectrons received from the sample into energy regions and, based on the total intensity in each region, determines the concentration of the element. The energy region corresponding to the elements Ti, V, Cr, MS, Fe, Co, Ni, Cu, Nb, Mo, Zn, Se, Zr, Ag, Sn, Ta, W, Au, Pb, Bi, Hf can be effectively analyzed.
The RF analyzer consists of a central processor, an X-ray tube, a detector, and an electronic memory that stores calibration data. In addition, the memory is also used to store and process alloy grade data and other coefficients related to various special operating modes.
As a rule, control over the study is carried out through a computer program based on a handheld portable computer (PDA), which provides the user with an image of the spectrum and the obtained element content values.
After the analysis, the values are compared with a database of steel grades and the closest grade is searched.
Scope of application
Metallographic control is mandatory for those types of equipment where metal is exposed to high temperatures, critical pressure and aggressive environments. This includes installations, apparatus, pipelines and containers in the energy, oil and gas, chemical and nuclear industries. There are at least a dozen GOSTs that establish regulatory characteristics and procedures for the use of metallography, and an even larger number of industry instructions, methods and regulations. For example, metallographic methods for assessing the grain size of steel in high-pressure steam pipelines at power plants (t up to 600 ºC, P up to 200 atmospheres) are regulated by GOST-5639. And in the event of their emergency failure, the industry regulatory document prescribes mandatory metallographic control.
Have any of you ever encountered metallographic testing of welds? In what cases is this used and how do metallographic examinations relate to flaw detection? Please write about your experience in the comments to this article.
What is metallographic analysis
Metallographic studies should not only determine the physical and chemical properties of a metal sample, but also describe such operational characteristics of its material as hardness, ductility, strength parameters, corrosion resistance, etc. Metallographic methods make it possible to obtain all these characteristics by studying the composition and structure of polished metal samples under a microscope at high magnification.
In the case of non-destructive testing, metallographic examinations are carried out directly on the product, for which portable optical equipment is used. During destructive testing, samples are cut out from the analyzed area of a part or workpiece, from which metallographic sections are then made - thin plates with a perfectly polished surface.
Most often, metallography is used in the study of samples made of steel and cast iron, which is due to the characteristics of the physical and metallurgical structure of these materials. Another area where metallography is widely used is the analysis of special alloys made of non-ferrous metals: titanium, tantalum, zirconium, etc. In addition, not a single examination of pipelines and metal structures that have been damaged as a result of accidents and disasters can be done without metallographic studies.