- Characteristics
- Areas of application
- Operating rules
- Anti-rust application
- Application for metal
Often metal and products made from it are subject to a characteristic “disease”, which manifests itself in the form of a red coating that corrodes the metal. We're talking about rust. Its formation occurs due to the effect of carbon dioxide, oxygen and water on the surface of a metal product. Of course, in order to extend the life of a metal product, it is necessary to begin the fight against corrosion as soon as possible. Treatment with phosphoric acid can help with this.
Physical properties
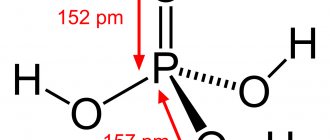
Phosphoric (orthophosphoric) acid with a molar mass of 97.99 g/mol and the empirical formula H3PO4 is an inorganic tribasic acid of medium strength. The structural formula of the molecule in the gaseous aggregate state is described in the form of a tetrahedron, containing a phosphorus atom in the center, and an oxygen atom and three hydroxyl groups at the vertices.
The composition is as follows:
| Name | Number of atoms | Mass fraction, % |
| Hydrogen (H) | 3 | 3,10 |
| Phosphorus (P) | 1 | 65,30 |
| Oxygen (O) | 4 | 31,60 |
Under normal conditions, the colorless crystals are hygroscopic, melt in air already at 42.35 °C, and easily dissolve in water, ethyl alcohol and other solvents. Three types of aqueous solutions have practical application:
| Concentration, % | Melting point, °C | Density, gram/ml |
| 75 | -20 | 1,579 |
| 80 | 0 | 1,633 |
| 85 | +20 | 1,689 |
A colorless and odorless syrupy liquid with an 85% concentration of H3PO4 is usually called orthophosphoric acid, and by boiling in a vacuum at 80 °C the anhydrous component is released from it. In the solid phase and in highly concentrated solutions, phosphoric acid molecules form intermolecular hydrogen bonds.
When diluting, hydrogen bonds between phosphate anions PO43- and water molecules H2O come to the fore.
Transportation rules
There are special GOSTs that stipulate the rules for transporting phosphoric acid, which is classified as dangerous goods. The substance can be delivered by any type of transport. The chemically active liquid is transported in tightly closed:
- steel tank trucks;
- bottles made of polyethylene, glass;
- plastic cubes;
- barrels;
- cans;
- rubberized railway tanks.
Chemical properties
H3PO4 solutions have different ionic compositions, depending on the acidity (pH) of the medium. As for all medium-strong tribasic acids, the electrolytic dissociation of phosphoric acid is three-stage, in the first stage the reaction is exothermic and is accompanied by the release of heat, and in the second and third it is endothermic:
- H3PO4 = H+ + H2PO4-.
- H2PO4- = H+ + HPO42-.
- HPO42- = H+ + PO43-.
Accordingly, salts are both medium - phosphates, and acidic - hydrophosphates and dihydrogen phosphates.
At room temperature, H3PO4 behaves quite inertly; when heated, it exhibits moderately acidic properties and changes the color of the indicators to red. It reacts with metals in the activity series up to hydrogen: 3Al + 2H3PO4 = Al3 (PO4)2 + 3H2. Enters into neutralization reactions with hydroxides: 3NaOH + H3PO4 = Na3PO4 + 3H2O; in the exchange reaction - with basic oxides: 3MgO + 2H3PO4 = Mg3 (PO4)2 + 3H2O.
Heating above 80 °C promotes interaction with passive oxides and silicates. Therefore, the phosphating process is widely used in metallurgy: a protective film of phosphates is formed on the surface of cast iron, steel or copper products, improving their characteristics. An increase in temperature leads to dehydration of the molecule with the formation of pyrophosphoric and metaphosphoric acid:
- 2H3PO4 = H2O + H4P2O7;
- H4P2O7 = H2O + 2HPO3.
Further heating increases the chain length, and as a result, polyphosphoric acids (HPO3) n with a polymeric structure are formed. Orthophosphoric acid alone reacts with silver nitrate, forming a bright yellow precipitate, while the others give a white one: H3PO4 + 3AgNO3 = Ag3PO4 + 3HNO3. Therefore, the precipitation of silver phosphate serves as a qualitative reaction to the phosphate ion.
Global market
Many developing countries have begun to focus on increasing phosphate mining and phosphate production. Some governments have already collaborated with various suppliers around the world to set up mineral extraction plants to produce phosphoric acid. In 2022, the global phosphoric acid market was valued at $45.85 billion. It is expected to reach over $61 billion by 2027, with a compound annual growth rate of 3.7%.
The market is segmented into Asia Pacific, Europe, North America, Latin America, and the Middle East and Africa. The Asia Pacific region is expected to dominate the global market in the near future. Phosphoric acid market growth in these regions will be further fueled by the developed agricultural sector in India and China.
Main methods of obtaining
For the first time, back in 1694, the English chemist Robert Boyle managed to synthesize phosphoric acid using phosphorus (V) oxide. A simple method of oxidizing phosphorus with dilute nitric acid is still widely used in laboratories today: 3P + 5NO3 + 2H2O = 3H3PO4 + 5NO. Heating anhydrous phosphorous acid to a boil leads to its decomposition into poisonous phosphine gas and orthophosphoric acid: 4H3PO3 = 3H3PO4 + PH3.
Two production options are of industrial importance: thermal and extraction. The first is the oxidation of elemental phosphorus during combustion to oxide (V): P4 + 5O2 = P4O10; and processing the final product with water: P4O10 + 6H2O = 4H3PO4.
Technically, this is implemented in various ways, named after the abbreviation of the patenting companies:
- The IG process combines both reactions in a single column made of low carbon percentage stainless steel. Phosphorus is supplied from above using compressed air or steam and burns at temperatures above 2000 °C. The reaction product, phosphorus (V) oxide, is absorbed by orthophosphoric acid, which flows evenly down the walls of the column. It simultaneously performs several important functions: dissolving P2O5, removing heat from the combustion zone, protecting walls from flames. The finished acid is collected at the bottom, cooled in a heat exchanger and returned to the column. The product of the IG process practically does not contain lower phosphorus compounds, but requires the removal of impurity arsenic, which always contaminates any phosphorus. Hydrogen sulfide solves this problem: it is released when sodium sulfide is introduced into a solution and precipitates arsenic sulfide, followed by filtration.
- The TVA process involves separating the combustion process of phosphorus from the absorption of its oxide. In a steel combustion chamber with external cooling, phosphorus combines with air, then the reaction products enter the absorption chamber, where they become phosphoric acid.
- The Hoechst process also carries out combustion and absorption separately, but utilizes the heat of the combustion reaction to generate working steam.
With the extraction method of production in Russia, natural phosphates (apatite concentrates from Khibiny or Karatau phosphorites) are treated with aqueous solutions of inorganic acids. This allows us to meet the country’s growing needs for mineral fertilizers. The resulting calcium sulfate adds a different number of water molecules depending on the conditions, and according to these characteristics, extraction processes are divided into several types:
- Dihydrate (CaSO4 2H2O). The raw material is crushed and fed into the reactor at a temperature of 70 to 80 °C separately from sulfuric acid. The concentration of the finished product is approximately 30%, and calcium sulfate is obtained in the form of dihydrate. Advantages: relatively low temperature to avoid corrosion; variety of phosphates used; processing large quantities. Disadvantages: the starting raw material requires preliminary preparation (grinding), and the resulting product requires additional concentration.
- Hemihydrate (CaSO4 0.5H2O). They are carried out at higher temperatures (from 80 to 100 °C), which makes it possible to obtain a stable form of crystalline hydrate - calcium sulfate hemihydrate. Phosphoric acid has a concentration of 40 to 48% and does not require additional processing.
- Combined hemihydrate-dihydrate processes are the merit of Japanese scientists. The raw material is processed at high temperatures, and the resulting hemihydrate recrystallizes into a dihydrate. The result is almost pure gypsum, a by-product of the reaction. It successfully meets the needs of the state economy, which does not have its own deposits.
Precipitation of anhydrous salt (anhydrite method) is theoretically feasible, but is not used industrially because it causes serious corrosion problems.
To concentrate the dihydrate product, vacuum evaporation is used, sometimes in several devices installed in series. This not only saves coolant, but also removes fluorine-containing impurities that are used in the production of hydrogen hexaphotosilicate H2SiF6. Other inorganic contaminants, arsenic and cadmium compounds, are removed by precipitation and extraction, and pure acid is freed from the solvent by distillation.
History of discovery
The discovery of phosphoric acid occurred in the 17th century in several independent laboratories.
The first mention of the discovery of phosphorus dates back to the German scientist Henning Brandt. A bankrupt entrepreneur, carried away by alchemical experiments in search of the “philosopher’s stone,” subjected the products of human activity to careful study. By evaporating the urine and heating the remaining sediment, Brandt obtained a white substance. Further experiments with this substance, which involved heating without oxygen, helped to isolate a fusible substance with unpleasant odors and vapors that glow in the dark. The first name of this substance had a very mystical character. “Cold fire,” that’s what Hening Brand called his discovery.
In the laboratory of the Anglo-Irish chemist Robert Boyle, known in scientific circles for preferring experience rather than speculation, phosphorus was isolated in 1680 using experiments with indicators. Boyle (with Kraft's advice) after conducting a series of experiments with liquids, blood, urine, hair and bones, put the production of phosphorus on a commercial basis.
The production of phosphorus in the 17th century was very successful commercially, due to its connection with alchemical searches, but in the 18th century, due to the discovery of another method of production (by the scientist Marggrave Andreas Sigismund) and its comprehensive disclosure, it ceased to have commercial appeal.
Areas of product use
Many sectors of the national economy have appreciated the properties of phosphoric acid. Its applications are surprisingly versatile - from scientific research in molecular biology to providing refrigerants for refrigeration units.
The production of mineral fertilizers consumes the lion's share of extraction acid, and more than 90% of phosphorus-containing ores are consumed here annually. Plants need phosphorus for the formation of seeds and fruits; its addition increases resistance to frost and drying out, which is especially important for northern regions with a short growing season and poor development of soil microorganisms.
The food industry has become interested in the antioxidant and stabilizing properties of phosphoric acid and has successfully used them in the E338 additive . It prevents rancidity, regulates acidity and extends shelf life, and adds flavor to syrups, soda, marmalade, bread and other baked goods. Debates about the dangers and benefits of such components have been going on for many years, but no one has yet proposed an alternative, and so far it all comes down to reasonable consumption.
Metalworking widely uses phosphoric acid as a flux when soldering copper, ferrous metals and stainless steel. Cleaning surfaces from rust is also very effective - a protective film is formed that prevents further corrosion.
Organic synthesis uses H3PO4 as a catalyst, the aviation industry has included it in hydraulic fluids, and woodworking impregnates wood, making it non-flammable. This track record includes fur farming, sucrose clarification and drug production, the production of fire-resistant impregnations and dentistry, where phosphoric acid is used to etch dental tissues before filling.
And also - the production of activated carbon, fire-resistant glass and ceramics, fire-retardant paints and varnishes, fire-resistant phosphate foam and particle boards. Phosphate salts are used to soften hard tap water and are included in SMS and descaling products.
Anti-rust application
A rust converter based on phosphoric acid creates a protective layer on the surface that protects against corrosion during further use. The peculiarity of using the compound is that it is safe for metal when applied. There are several ways to remove rust with phosphoric acid, depending on the size of the damage:
- etching with immersion in a bath or other container;
- repeated application of the composition to the metal with a spray gun or roller;
- covering the surface with pre-treated mechanical cleaning.
The orthophosphorus compound converts rust into iron phosphates. The composition can be used for washing and cleaning:
- rolled metal products;
- wells;
- pipeline surfaces;
- steam generators;
- water supply, heating systems;
- coils;
- boilers;
- water heaters;
- heat exchangers;
- boilers;
- machine parts and mechanisms.
Ecology and safety
Using the extraction method, which is the least energy-intensive, up to 95% of the total amount of acid is obtained, and the remaining 5% comes from the thermal method. The main producer and consumer of extraction H3PO4 is the USA (about 90% of global volumes), followed by Russia and Morocco. Dumps of contaminated calcium sulfate formed during the extraction method need to be disposed of.
Today they are dumped on land, flooded in reservoirs, and only a small part is used as raw material for processing. The reduction in production in the 80s of the last century was caused by the abandonment of phosphorus-containing solvents and mineral fertilizers that pollute groundwater.
Orthophosphoric acid has no specific effect, has weak systemic toxicity and, in terms of the degree of impact on the human body, belongs to the second hazard class according to GOST 12.1.005. When the concentration increases, its vapor causes changes in the mucous membranes and crumbling of teeth, as well as skin inflammation.
Contact has an irritating effect at a solution concentration of up to 10%, and above 25% it is corrosive and burns.
Working with the drug requires the use of personal protective equipment (respirator, rubber gloves, special glasses) and compliance with personal hygiene rules. Ingestion of large quantities causes nausea, diarrhea and vomiting. To eliminate the consequences, the skin and eyes are washed with warm water or saline and fluid losses are replenished intravenously.
Rules for working with acid
Work with phosphoric acid should take place in a room with good ventilation. Be sure to wear a respirator to prevent caustic vapors from entering your respiratory tract and goggles to protect your eyes. If you do not have these protective equipment at hand, you should use a mask. Be sure to wear gloves and protect the exposed skin from contact with the substance, as this can lead to burns. If it gets on your skin, rinse it thoroughly under running water and then seek medical help.
Transportation of the reagent must be carried out in special transport equipped with iron tanks that are not susceptible to its destructive influence. It can also be transported using other vehicles, both land and water. But at the same time, all safety regulations must be observed.
It should be stored in a place protected from direct sunlight. The shelf life under such conditions is no more than 1 year.
Crystal lattice:
| 300 | Crystal cell |
| 311 | Crystal grid #1 |
| 312 | Lattice structure |
| 313 | Lattice parameters |
| 314 | c/a ratio |
| 315 | Debye temperature |
| 316 | Name of space symmetry group |
| 317 | Symmetry space group number |